Aptazapine
- CAS NO.:71576-40-4
- Empirical Formula: C16H19N3
- Molecular Weight: 253.34
- MDL number: MFCD00865377
- SAFETY DATA SHEET (SDS)
- Update Date: 2022-12-21 16:56:50
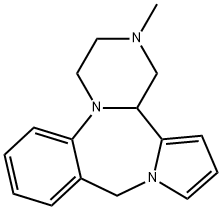
What is Aptazapine?
Originator
Aptazapine maleate,ZYF Pharm Chemical
The Uses of Aptazapine
Antidepressant.
Manufacturing Process
To the suspension of 12.8 g of 2-methyl-3,4-dioxo-1,3,4,14b-tetrahydro-10Hpyrazino[
1,2-a]pyrrolo[2,1-c][1,4]benzodiazepine in 460 ml of
tetrahydrofuran, 200 ml of 1-molar diborane in tetrahydrofuran are added
while stirring and cooling with ice. The mixture is refluxed for one hour, again
cooled and combined with 25 ml of acetic acid. It is evaporated, the residue
taken up in 50 ml of 30% aqueous sodium hydroxide and the mixture
extracted with methylene chloride. The extract is dried, evaporated, the
residue dissolved in diethyl ether, the solution filtered and the filtrate
evaporated, to yield the crude 2-methyl-1,3,4,14b-tetrahydro-10Hpyrazino[
1,2-a]pyrrolo[2,1-c][1,4]benzodiazepine. It is triturated with ethyl
acetate-diethyl ether, chromatographed on 70 g of silica gel and eluted with
methanol-chloroform (1:9). The eluate is evaporated and the residue salified.
9.7 g thereof are dissolved in the minimum amount of isopropanol and the
solution acidified with a concentrated solution of 4.45 g of maleic acid is
isopropanol. The precipitate formed is collected and recrystallized from
methanol-diethyl ether, to yield the corresponding mono-maleate melting at
180°-182°C.
The starting material is prepared as follows: the mixture of 54.0 g of Npotassium
phthalimide, 50.0 g of o-nitrobenzyl chloride and 120 ml of
dimethylformamide is refluxed for 3 hours and poured into 900 ml of icewater
while stirring. After 30 minutes, it is filtered, and the residue washed
with water, to yield the N-o-nitrobenzyl-phthalimide melting at 190°-209°C.
The mixture of 70.0 g thereof, 14.6 g of hydrazine hydrate and 600 ml of
ethanol is refluxed for 4 hours and combined with 50 ml of concentrated
hydrochloric acid. After 30 minutes, it is cooled to room temperature, filtered
and the residue washed with water. The filtrate is concentrated, the aqueous
concentrate filtered and the filtrate basified with 3 N aqueous sodium
hydroxide. It is extracted with diethyl ether, the extract dried and evaporated,
to yield the o-nitrobenzylamine.
To the solution of 7.6 g thereof in 25 ml of glacial acetic acid, 6.6 g of 2,5-
dimethoxytetrahydrofuran are added and the mixture is refluxed for one hour.
It is evaporated, the residue poured into ice water and the mixture extracted
with ethyl acetate. The extract is washed with saturated aqueous sodium
bicarbonate, dried and evaporated. The residue is taken up in diethyl ether,
the solution decolorized with charcoal, filtered and evaporated, to yield the 1-
(o-nitrobenzyl)pyrrole.
Through the mixture of 13.42 g thereof, 140 ml of diethyl ether and 6.85 ml
of chloroacetonitrile, hydrogen chloride is bubbled while stirring and cooling in an ice-salt bath. The saturated mixture is stirred at room temperature
overnight, filtered and the residue suspended in 100 ml of water. It is
extracted 3 times with 100 ml of ethyl acetate, warmed on the steam bath
while stirring until all is dissolved, and the solution evaporated, to yield the 1-
o-nitrobenzyl-2-chloroacetylpyrrole.
To the solution of 16.2 g thereof in 450 ml of ethanol, 14.10 g of N-methylbenzylamine
are added and the mixture is refluxed for 3 hours. It is
evaporated, the residue taken up in methylene chloride, the solution washed
with saturated aqueous sodium carbonate dried, filtered and evaporated. The
residue is triturated with diethyl ether, to yield the 1-(o-nitrobenzyl)-2-(Nmethyl-
N-benzylaminoacetyl)pyrrole.
The solution of 3.0 g thereof in 30 ml of glacial acetic acid is hydrogenated
over 100 mg of platinum oxide at 2.7 atm and room temperature until the
theoretical amount of hydrogen has been absorbed. It is filtered, the filtrate
evaporated, the residue taken up in methylene chloride-diethyl ether and the
solution washed with saturated aqueous sodium bicarbonate. It is dried,
evaporated, the residue chromatographed on 30 g of silica gel and eluted with
methanol-chloroform (1:9), to yield the 11-(N-methyl-N-benzylaminomethyl)-
10,11-dihydro-5H-pyrrolo[2,1-c][1,4]benzodiazepine melting at 147°-149°C.
The solution of 500 mg thereof in 35 ml of ethanol and 5 ml of glacial acetic
acid is hydrogenated over 250 mg of 5% palladium on charcoal at 2.7 atm
and 40°C for 7 hours. The mixture is filtered, the filtrate evaporated and the
residue taken up in methylene chloride. The solution is washed with saturated
aqueous sodium carbonate, the aqueous phase extracted with methylene
chloride and the combined organic solutions dried and evaporated, to yield the
11-(N-methylaminomethyl)-10,11-dihydro-5H-pyrrolo[2,1-
c][1,4]benzodiazepine.
The mixture of 300 mg thereof and 232 mg of diethyl oxalate is slowly heated
to 140°C during 45 minutes and to 180°C during 15 minutes, at which
temperature it is maintained for 30 minutes. It is cooled, diluted with
benzene, chromatographed on silica gel and eluted with methanol-chloroform
(1:9), to yield the 2-methyl-3,4-dioxo-1,3,4,14b-tetrahydro-10H-pyrazino[1,2-
a]pyrrolo[2,1-c][1,4]benzodiazepine melting at 178°-179°C.
In practice it is usually used as maleate salt.
Therapeutic Function
Antidepressant
Properties of Aptazapine
| Boiling point: | 432.6±45.0 °C(Predicted) |
| Density | 1.22±0.1 g/cm3(Predicted) |
| pka | 8.44±0.20(Predicted) |
Safety information for Aptazapine
Computed Descriptors for Aptazapine
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

![1-[2-(4-Methylpiperazin-1-yl)phenyl]methanamine](https://img.chemicalbook.in/CAS/GIF/655256-68-1.gif)
![10,11-DIHYDRO-5H-BENZO[E]PYRROLO[1,2-A][1,4]DIAZEPINE](https://img.chemicalbook.in/CAS/GIF/22162-53-4.gif)

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4