APHIDICOLIN
Synonym(s):APC;Aphidicolin - CAS 38966-21-1 - Calbiochem;ICI 69653;InSolution Aphidicolin - CAS 38966-21-1 - Calbiochem;NSC-234714
- CAS NO.:38966-21-1
- Empirical Formula: C20H34O4
- Molecular Weight: 338.48
- MDL number: MFCD00083214
- EINECS: 609-602-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
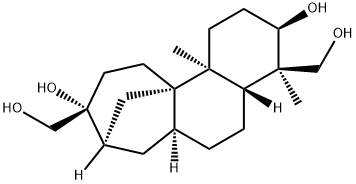
What is APHIDICOLIN?
Description
(+)-
Chemical properties
White crystal
The Uses of APHIDICOLIN
(+)-Aphidicolin is a naturally occurring tetracyclic diterpene with potential antiviral and antimitotical properties.
The Uses of APHIDICOLIN
Aphidicolin is a tetracyclic diterpene antibiotic isolated from fungi, notably Cephalosporium, Nigrospora, Harziella and Phoma. Aphidicolin has antibiotic, antiviral and antimitotic properties, blocking the cell cycle at early S-phase. This property has been used to synchronise cell division and is useful as a tool in cell differentiation research. Aphidicolin is a reversible inhibitor of DNA replication by inhibiting selected DNA polymerases. Aphidicolin induces apoptosis, prolongs the half-life of DNA methyltransferase, is active against Leishmania parasites and acts synergistically with the antitumour agents, vincristine and doxorubicin.
The Uses of APHIDICOLIN
A DNA polymerase inhibitor. Blocks the cell cycle at the early S-phase
What are the applications of Application
Aphidicolin is a novel tetracyclic diterpene antibiotic and also a DNA synthesis inhibitor in eukaryotes.
Definition
ChEBI: A tetracyclic diterpenoid that has an tetradecahydro-8,11a-methanocyclohepta[a]naphthalene skeleton with two hydroxymethyl substituents at positions 4 and 9, two methyl substituents at positions 4 and 11b and two hydroxy substituents at positi ns 3 and 9. An antibiotic with antiviral and antimitotical properties. Aphidicolin is a reversible inhibitor of eukaryotic nuclear DNA replication.
General Description
A cell-permeable tetracyclic diterpene antibiotic. Cell synchronization agent. Blocks the cell cycle at the early S-phase. Specific inhibitor of DNA polymerase α and δ in eukaryotic cells and in some viruses of animal origin. Potentiates apoptosis induced by arabinosyl nucleosides in leukemia cell lines. Also induces apoptosis in HeLaS3 cells, but inhibits vincristine-induced apoptosis in the p53-negative human prostate cancer cell line PC-3.
Biochem/physiol Actions
Cell permeable: yes
storage
+4°C
References
1) Syvaoja et al. (1990), DNA polymerases alpha, delta, and epsilon: three distinct enzymes from HeLa cells; Proc. Natl. Acad. Sci. USA, 87 6664 2) Urbani et al. (1995), Dissociation of nuclear and cytoplasmic cell cycle progression by drugs employed in cell synchronization; Exp. Cell Res., 219 159 3) Kuwakado et al. (1993), Aphidicolin potentiates apoptosis induced by arabinosyl nucleosides in human myeloid leukemia cell lines; Biochem. Pharmacol., 46 1909 4) Yin and Schimke (1996), Inhibition of apoptosis by overexpressing Bcl-2 enhances gene amplification by a mechanism independent of aphidicolin pretreatment; Proc. Natl. Acad. Sci. USA, 93 3394
Properties of APHIDICOLIN
| Melting point: | 218-220°C |
| Boiling point: | 394.61°C (rough estimate) |
| alpha | D27 +12° (c = 1 in methanol) |
| Density | 1.0057 (rough estimate) |
| vapor pressure | 0.55 hPa ( 20 °C) |
| refractive index | 1.4434 (estimate) |
| Flash point: | 87℃ |
| storage temp. | 2-8°C |
| solubility | ethanol: soluble1mg/mL (stable at least a week at 4°C.) |
| form | White solid |
| pka | 14.24±0.70(Predicted) |
| color | colorless |
| optical activity | [α]27/D +12°, c = 1 in methanol(lit.) |
| Water Solubility | Soluble in DMSO or methanol. Insoluble in water. Store solutions at -20° |
| Merck | 13,734 |
| BRN | 4689958 |
| Stability: | Stable for 2 years from date of purchase from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
Safety information for APHIDICOLIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for APHIDICOLIN
APHIDICOLIN manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Aphidicolin CAS 38966-21-1View Details
Aphidicolin CAS 38966-21-1View Details
38966-21-1 -
 Aphidicolin, Ready Made Solution CAS 38966-21-1View Details
Aphidicolin, Ready Made Solution CAS 38966-21-1View Details
38966-21-1 -
 Aphidicolin CAS 38966-21-1View Details
Aphidicolin CAS 38966-21-1View Details
38966-21-1 -
 Aphidicolin CAS 38966-21-1View Details
Aphidicolin CAS 38966-21-1View Details
38966-21-1 -
 Aphidicolin from Nigrospora sphaerica CAS 38966-21-1View Details
Aphidicolin from Nigrospora sphaerica CAS 38966-21-1View Details
38966-21-1 -
 38966-21-1 (+)-Aphidicolin 98%View Details
38966-21-1 (+)-Aphidicolin 98%View Details
38966-21-1 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1