7783-70-2
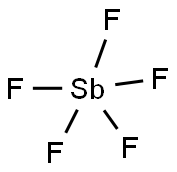
Product Name:
ANTIMONY PENTAFLUORIDE
Formula:
F5Sb
Synonyms:
Antimony pentafluoride;Antimony(V) fluoride
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Antimony pentafluoride appears as a colorless, oily liquid. Fumes irritate the eyes and mucous membranes. Toxic. Corrosive to metals and tissue. Extremely dangerous to tissue; its burns may be followed by gangrene. Only shipped in cylinders. Under prolonged exposure to heat cylinders may violently rupture and rocket. Used to make other chemicals as well as a catalyst in the manufacture of other chemicals. |
|---|---|
| Color/Form | Hygroscopic, moderately viscous liquid |
| Odor | Sharp odor |
| Boiling Point | 286 °F at 760 mmHg (EPA, 1998) |
| Melting Point | 47 °F (EPA, 1998) |
| Solubility | Reacts violently with water |
| Density | 3.097 at 78.44 °F (EPA, 1998) - Denser than water; will sink |
| Vapor Pressure | Vapor pressure, kPa at 25 °C: 1.33 |
| Stability/Shelf Life | Stable under recommended storage conditions. |
| Decomposition | Hazardous decomposition products formed under fire conditions - Hydrogen fluoride, antimony oxide. |
| Corrosivity | Slowly corrodes glass, copper, lead |
| Surface Tension | 20 dynes/cm = 0.20 N/m at 20 °C |
| Other Experimental Properties | Very reactive |
| Chemical Classes | Toxic Gases & Vapors -> Other Toxic Gases & Vapors |
SAFETY INFORMATION
| Signal word | Danger |
|---|---|
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 216.752 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 0 |
| Exact Mass | 215.89583 g/mol |
| Monoisotopic Mass | 215.89583 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 6 |
| Formal Charge | 0 |
| Complexity | 37.1 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Antimony pentafluoride appears as a colorless, oily liquid. Fumes irritate the eyes and mucous membranes. Toxic. Corrosive to metals and tissue. Extremely dangerous to tissue; its burns may be followed by gangrene. Only shipped in cylinders. Under prolonged exposure to heat cylinders may violently rupture and rocket. Used to make other chemicals as well as a catalyst in the manufacture of other chemicals.
