Anthraquinone
Synonym(s):9,10-Dihydro-9,10-anthracenedione;Anthraquinone;ATQ
- CAS NO.:84-65-1
- Empirical Formula: C14H8O2
- Molecular Weight: 208.21
- MDL number: MFCD00001188
- EINECS: 201-549-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 11:34:44
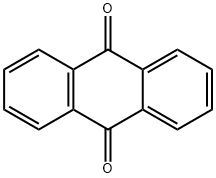
What is Anthraquinone?
Description
Anthraquinone is a combustible, light yellowto green crystalline solid. Molecular weight= 208.23;Boiling point= 380℃; Melting/Freezing point= 286℃(sublimes); Flash point= 185℃ (cc). NFPA 704 M HazardIdentification (based on NFPA-704 M Rating System):Health 0, Flammability 1, Reactivity 0. Very slightly solublein water; solubility=,13 mg/L at 22℃.
Chemical properties
Anthraquinone is a combustible, light yellow to green crystalline solid. Soluble in ethanol, ether and acetone, insoluble in water. It may be prepared by reacting benzene with phthalic anhydride. The compoundis the basis of a range ofdyestuffs.
Occurrence
Anthraquinones naturally occur in some plants (eg. aloe, senna, rhubarb, and Cascara buckthorn), fungi, lichens, and insects, where they serve as a basic skeleton for their pigments. Natural anthraquinone derivatives tend to have laxative effects.
The Uses of Anthraquinone
Anthraquinone is used in paper industry as a catalyst to increase the pulp production yield and to improves the fiber strength through reduction reaction of cellulose to carboxylic acid. It is also used as a precursor for dye formation.
What are the applications of Application
Anthraquinone is a precursor for dye formation
Definition
ChEBI: Anthraquinone is an anthraquinone that is anthracene in which positions 9 and 10 have been oxidised to carbonyls. It is a colorless crystalline quinone used in producing dyestuffs such as alizarin.
Preparation
Anthraquinone is obtained by oxidation of anthracene using sodium dichromate plus sulfuric acid, and is purified by dissolving in concentrated sulfuric acid at 130 °C and pouring into boiling water, whereupon anthraquinone separates as pure solid, and is recovered by filtration. Further purification may be accomplished by sublimation or crystallization from nitrobenzene, aniline or tetrachloroethane.
Synthesis Reference(s)
Journal of the American Chemical Society, 102, p. 1457, 1980 DOI: 10.1021/ja00524a059
The Journal of Organic Chemistry, 29, p. 987, 1964 DOI: 10.1021/jo01027a538
Tetrahedron Letters, 24, p. 5499, 1983 DOI: 10.1016/S0040-4039(00)94122-4
General Description
Anthraquinone is a polycyclic aromatic hydrocarbon derived from anthracene or phthalic anhydride. Anthraquinone is used in the manufacture of dyes, in the textile and pulp industries, and as a bird repellant.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Anthraquinone is incompatible with strong oxidizing agents.
Hazard
Possible carcinogen.
Fire Hazard
Anthraquinone is combustible.
Flammability and Explosibility
Non flammable
Agricultural Uses
Repellent, Seed treatment: Used as a seed dressing or protectant. Banned in EU.
Trade name
(p)ANTHRAPEL®; FLIGHT CONTROL- PLUS®; HOELITE®; MORKIT®; REPELL®
Pharmacology
Anthraquinone is a secondary repellent and affects birds by causing post-ingestional distress (40). Sometimes, ingestion of anthraquinone-treated food produces vomiting, but often vomiting does not occur and the bird just sits quietly until the discomfort passes. Unlike methiocarb, anthraquinone doe not affect the bird’s nervous system and does not immobilize affected birds. Presumably, the emetic response is produced through irritation of the gut lining, but the actual mechanism is unclear. It is clear, however, that anthraquinone is not a taste repellent or contact irritant. Birds do not hesitate to eat treated food, and they exhibit no sign that treated food is unpalatable to them. The post-ingestional discomfort that results from eating anthraquinone-treated food produces a conditioned aversion to that food type. Birds need to experience the adverse consequences before learning to avoid the protected food. Thus, it is not reasonable to expect losses to cease immediately upon application of the repellent. There will be some level of loss in the crop as the depredating birds acquire the learned avoidance response.
Safety Profile
Moderately toxic by intraperitoneal route. A mild allergen. Mutation data reported. Combustible when exposed to heat or flame. To fight fire, use water, foam, CO2, water spray or mist, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.
Toxicology
Based on present knowledge, anthraquinone, unlike
the “emodins” (hydroxyanthraquinone glycosides)
from anthraquinone drugs , is biologically
completely “inert,” i.e., inactive, presumably
as a consequence of its insolubility in
water and lipids.
There is no toxicologic information about
anthraquinone. Even in the Toxic Substances List
no LD50 is found for anthraquinone. In contrast
to benzoquinone, which causes severe local
irritation and is included in the list of occupational
hazards because of its damaging effect on
the cornea, a fine dust of anthraquinone to which
emulgators were added had no greater effect on
the eyes of rabbits than a talcum suspension, as
was shown in a BASF study.
An MAK value of 10 mg/m3 was recommended
; therefore, anthraquinone dust is
considered the same as inert dust.
Synthesis
Anthraquinone is produced from anthracene,
where this is available from coal tar, either by oxidation
with chromic acid in 48 % sulfuric acid
or by oxidation with air in the vapor phase. The
oxidation with chromic acid is competitive, provided
that the chromium(III) sulfate lye formed
can be processed to tanning agents. Anthracene
with purity of '94 % is required for both oxidation
processes; crude anthracene from coal tar
must be purified by recrystallization.
About 85 % of world production is based
on the oxidation of anthracene. Since the mid-
1970s, anthracene production has fallen continuously,
creating a supply shortage. Therefore, the
complex naphthalene process is gaining in importance.
There is an adequate supply of naphthalene
in coal tar. If necessary, additional naphthalene
can be isolated from the residual oils of
gasoline reforming, a process common in the
United States.
Potential Exposure
Anthraquinone is an important starting material for vat dye manufacture. Also used in making organics; and used as a bird repellent in seeds.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions,including resuscitation mask) if breathing has stopped andCPR if heart action has stopped. Transfer promptly to amedical facility. When this chemical has been swallowed,get medical attention. Give large quantities of water andinduce vomiting. Do not make an unconscious person vomit.
Storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Store in tightly closed containers in a cool, well-ventilatedarea away from oxidizers. Sources of ignition, such as smoking and open flames, are prohibited where this chemical isused, handled, or stored in a manner that could create apotential fire or explosion hazard.
Shipping
UN3143 Dyes, solid, toxic, n.o.s. or Dye intermediates, solid, toxic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Crystallise anthraquinone from CHCl3 (38mL/g), *benzene, or boiling acetic acid, wash it with a little EtOH and dry it under vacuum over P2O5. [Beilstein 7 IV 2556.]
Toxicity evaluation
Anthraquinone is a stable compound that is virtually insoluble in water. It is not phytotoxic and does not inhibit germination of rice seeds or growth of sprouts. It has very low toxicity to birds and mammals, and it appears to be innocuous to insects as well. There is no known hazard to nontarget species from repellent applications of Flight Control.
Incompatibilities
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides
Waste Disposal
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed.
Properties of Anthraquinone
| Melting point: | 284-286 °C (lit.) |
| Boiling point: | 379-381 °C (lit.) |
| Density | 1.438 |
| vapor density | 7.16 (vs air) |
| vapor pressure | 1 mm Hg ( 190 °C) |
| refractive index | 1.5681 (estimate) |
| Flash point: | 365 °F |
| storage temp. | no restrictions. |
| solubility | 0.00007g/l |
| form | Powder |
| color | Yellow-green to khaki to tan |
| Water Solubility | <0.1 g/100 mL at 23 ºC |
| Merck | 14,687 |
| BRN | 390030 |
| Stability: | Stable. Incompatible with strong oxidizing agents. Combustible. |
| CAS DataBase Reference | 84-65-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 9,10-Anthraquinone(84-65-1) |
| IARC | 2B (Vol. 101) 2013 |
| EPA Substance Registry System | Anthraquinone (84-65-1) |
Safety information for Anthraquinone
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H350:Carcinogenicity |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Anthraquinone
| InChIKey | RZVHIXYEVGDQDX-UHFFFAOYSA-N |
Anthraquinone manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran

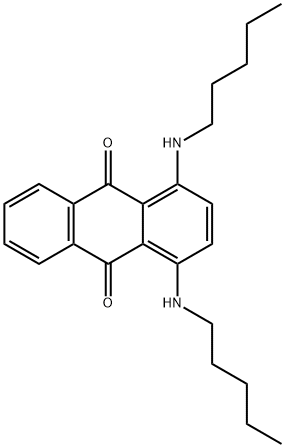
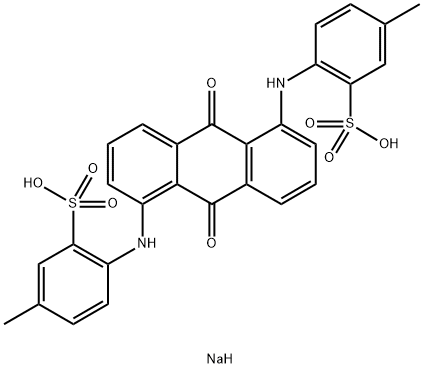

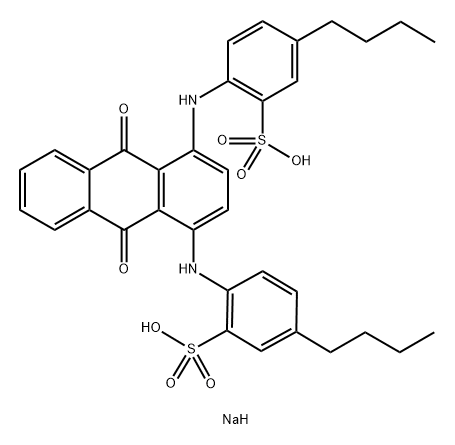
You may like
-
 84-65-1 98%View Details
84-65-1 98%View Details
84-65-1 -
 Anthraquinone pure CAS 84-65-1View Details
Anthraquinone pure CAS 84-65-1View Details
84-65-1 -
 Anthraquinone, GR 99%+ CAS 84-65-1View Details
Anthraquinone, GR 99%+ CAS 84-65-1View Details
84-65-1 -
 Anthraquinone CAS 84-65-1View Details
Anthraquinone CAS 84-65-1View Details
84-65-1 -
 Anthraquinone CAS 84-65-1View Details
Anthraquinone CAS 84-65-1View Details
84-65-1 -
 Anthraquinone 98% CAS 84-65-1View Details
Anthraquinone 98% CAS 84-65-1View Details
84-65-1 -
 ANTHRAQUINONE For Synthesis CAS 84-65-1View Details
ANTHRAQUINONE For Synthesis CAS 84-65-1View Details
84-65-1 -
 ANTHRAQUINONE 84 - 65 -1View Details
ANTHRAQUINONE 84 - 65 -1View Details
84-65-1
