ANTHRANILIC ACID CINNAMYL ESTER
- CAS NO.:87-29-6
- Empirical Formula: C16H15NO2
- Molecular Weight: 253.3
- MDL number: MFCD00053631
- EINECS: 201-738-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-06-12 17:01:53
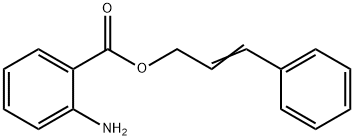
What is ANTHRANILIC ACID CINNAMYL ESTER?
Chemical properties
Water insoluble, reddish-yellow powder, soluble in ethanol, ethyl ether and chloroform.
Chemical properties
Cinnamyl anthranilate has a balsamic, fruity odor somewhat reminiscent of grape.
The Uses of ANTHRANILIC ACID CINNAMYL ESTER
Flavoring agent in food; fragrance in soaps and perfumes.
The Uses of ANTHRANILIC ACID CINNAMYL ESTER
Cinnamyl Anthranilate is a flavoring agent that is a powder which may be red or yellow. It has an odor resembling anthranilates, fruity and characteristically balsamic. It is insoluble in water, and soluble in alcohol, chloroform, and ether. It is obtained by chemical synthesis.
Preparation
By esterification of anthranilic acid with cinnamyl alcohol.
Definition
ChEBI: Cinnamyl anthranilate is a benzoate ester.
General Description
Brownish or reddish-yellow powder or light yellow solid with a fruity taste.
Air & Water Reactions
ANTHRANILIC ACID CINNAMYL ESTER is sensitive to oxidation and hydrolysis. Insoluble in water.
Reactivity Profile
ANTHRANILIC ACID CINNAMYL ESTER is sensitive to oxidation and hydrolysis. ANTHRANILIC ACID CINNAMYL ESTER is subject to photooxidation. ANTHRANILIC ACID CINNAMYL ESTER reacts with various aldehydes, forming highly colored compounds.
Hazard
Questionable carcinogen.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition ANTHRANILIC ACID CINNAMYL ESTER emits toxic fumes of nitrogen oxides.
Fire Hazard
Flash point data for ANTHRANILIC ACID CINNAMYL ESTER are not available; however, ANTHRANILIC ACID CINNAMYL ESTER is probably combustible.
Safety Profile
Suspected carcinogen with experimental carcinogenic and neoplastigenic data. Mutation data reported. See also ESTERS. Combustible liquid. When heated to decomposition it emits toxic fumes of NOx.
Synthesis
Anthranilic acid cinnamyl ester is prepared from Isatoic anhydride plus Cinnamic alcohol.
Properties of ANTHRANILIC ACID CINNAMYL ESTER
| Melting point: | 64-66°C |
| Boiling point: | 322°C |
| Density | 1,18 g/cm3 |
| refractive index | 1.6000 (estimate) |
| Flash point: | 322°C |
| pka | 2.15±0.10(Predicted) |
| color | Reddish-yellow powder |
| Odor | balsamic odor |
| Water Solubility | 0.23mg/L(temperature not stated) |
| Merck | 14,2303 |
| BRN | 2733435 |
| IARC | 3 (Vol. Sup 7, 77) 2000 |
| EPA Substance Registry System | Cinnamyl anthranilate (87-29-6) |
Safety information for ANTHRANILIC ACID CINNAMYL ESTER
Computed Descriptors for ANTHRANILIC ACID CINNAMYL ESTER
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


![2-[(E)-2-(3-CHLOROPHENYL)VINYL]-8-METHOXY-4H-3,1-BENZOXAZIN-4-ONE](https://img.chemicalbook.in/CAS/20200331/GIF/CB21516812.gif)
![6,8-DICHLORO-2-(2-(4-CHLORO-3-NITROPHENYL)VINYL)BENZO[D]1,3-OXAZIN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB1681259.gif)


![2-(2-(3-NITROPHENYL)VINYL)BENZO[D]1,3-OXAZIN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB8288332.gif)

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4