ANISOMYCIN
Synonym(s):(2R,3S,4S)-2-(4-Methoxybenzyl)-3,4-pyrrolidinediol-3-acetate;2-( p-Methoxybenzyl)-3,4-pyrrolidinediol-3-acetate;2-(p-Methoxybenzyl)-3,4-pyrrolidinediol-3-acetate;2-[(4-Methoxyphenyl)methyl]-3,4-pyrrolidinediol 3-acetate;Flagecidin
- CAS NO.:22862-76-6
- Empirical Formula: C14H19NO4
- Molecular Weight: 265.3
- MDL number: MFCD00077650
- EINECS: 245-269-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-05-29 17:48:59
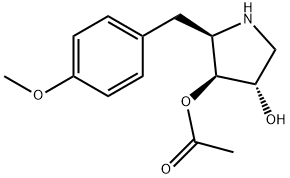
What is ANISOMYCIN?
Description
Anisomycin (22862-76-6) activates JNK/SAPKs and reduces c-fos and c-jun. Protein synthesis inhibitor. Anisomycin induces apoptosis and sensitizes cells to anoikis. Cell permeable.
Chemical properties
Crystalline
The Uses of ANISOMYCIN
Anisomycin is a phenylmethylenepyrrolidine first isolated from Streptomyces griseolus in 1954 as an antiprotozoan with antifungal activity. Anisomycin is an inhibitor of protein synthesis by binding to the 60S ribosomal subunit. Interestingly, anisomycin has found use for the induction of amnesia in animal models. More recently, anisomycin has been demonstrated to induce apoptosis, to be a selective signalling agonist, to activate mitogen-activated protein (MAP) kinases and to be immunomodulatory via its action on T cells.
The Uses of ANISOMYCIN
It is applied as an antiparasitic agent and antineoplastic agent. It acts as a protein synthesis inhibitor (blocks translation), potent activator of stress-activated protein kinases (JNK/SAPK) and p38 MAP kinase. It also scts as a potent signaling agonist to selectively elicit homologous desensitization of immediate early gene induction (c-fos, fosB, c-jun, junB and junD). Activates mitogen-activated protein (MAP) kinases (JNK/SAPK and p38/RK).
What are the applications of Application
Anisomycin is activator of JNK (c-Jun N-terminal kinases) and p38
Definition
ChEBI: An antibiotic isolated from various Streptomyces species. It interferes with protein and DNA synthesis by inhibiting peptidyl transferase or the 80S ribosome system.
Biological Activity
Protein synthesis inhibitor (blocks translation). Potent activator of stress-activated protein kinases (JNK/SAPK) and p38 MAP kinase. Acts as a potent signaling agonist to selectively elicit homologous desensitization of immediate early gene induction (c-fos, fosB, c-jun, junB and junD).
Biochem/physiol Actions
Antibiotic isolated from Streptomyces griseolus that inhibits protein synthesis. Acts by inhibiting peptidyl transferase activity in eukaryote ribosomes. Reported to induce apoptosis in a variety of cells including promyelocytic leukemia cells, Jurkat cells, ventricular myocytes, and colon adenocarcinoma cells. Initiates intracellular signals and immediate early gene induction. Selective signaling agonist. Potent Jun-NH2 terminal kinase (JNK) agonist. Activates mitogen-activated protein (MAP) kinases (JNK/SAPK and p38/RK). Antiprotozoal agent.
Safety Profile
Poison by ingestion,intraperitoneal, subcutaneous, and intravenous routes.When heated to decomposition it emits toxic fumes ofNOx.
storage
+4°C
References
1) Hazzalin?et al. (1998),?Anisomycin Selectively Desensitizes Signalling Components Involved in Stress Kinase Activation and fos and jun Induction; Mol. Cell. Bio.,?8?1844 2) Mawji?et al. (2007),?A Chemical Screen Identifies Anisomycin as an Anoikis Sensitizer That Functions by Decreasing FLIP Protein Synthesis; Cancer Res.,?67?8307
Properties of ANISOMYCIN
| Melting point: | 140-141 C |
| Boiling point: | 408.52°C (rough estimate) |
| alpha | D23 -30° (methanol) |
| Density | 1.1356 (rough estimate) |
| refractive index | 1.5230 (estimate) |
| Flash point: | 87℃ |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | methanol: 20 mg/mL, clear, colorless to faintly yellow |
| form | solid |
| pka | 7.9(at 25℃) |
| color | white |
| Water Solubility | Soluble in water at 2 mg/ml, in DMSO at 20mg/ml, and in methanol at 20mg/ml |
| λmax | 283nm(EtOH)(lit.) |
| Merck | 14,670 |
| BRN | 20705 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
Safety information for ANISOMYCIN
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for ANISOMYCIN
| InChIKey | YKJYKKNCCRKFSL-RDBSUJKOSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Anisomycin CAS 22862-76-6View Details
Anisomycin CAS 22862-76-6View Details
22862-76-6 -
 Anisomycin, ≥98% (HPLC) CAS 22862-76-6View Details
Anisomycin, ≥98% (HPLC) CAS 22862-76-6View Details
22862-76-6 -
 Anisomycin, Ready Made Solution from Streptomyces griseolus CAS 22862-76-6View Details
Anisomycin, Ready Made Solution from Streptomyces griseolus CAS 22862-76-6View Details
22862-76-6 -
 Anisomycin from Streptomyces griseolus CAS 22862-76-6View Details
Anisomycin from Streptomyces griseolus CAS 22862-76-6View Details
22862-76-6 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1