Anileridine
- CAS NO.:144-14-9
- Empirical Formula: C22H28N2O2
- Molecular Weight: 352.47
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-15 10:43:25
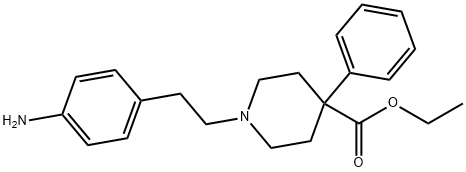
What is Anileridine?
Absorption
Anileridine is absorbed by all routes of administration.
Toxicity
Symptoms of overexposure include dizziness, perspiration, a feeling of warmth, dry mouth, visual difficulty, itching, euphoria, restlessness, nervousness and excitement have been reported.
Originator
Leritine HCl,Merck Sharp and Dohme,US,1958
The Uses of Anileridine
A synthetic analgesic drug. 4-phenylpiperidine derivative that is an analog of Meperidine (M223900) with increased analgesic activity.
Background
Anileridine is a synthetic opioid and strong analgesic medication. It is a narcotic pain reliever used to treat moderate to severe pain. Narcotic analgesics act in the central nervous system (CNS) to relieve pain. Some of their side effects are also caused by actions in the CNS.
Indications
For treatment and management of pain (systemic) and for use as an anesthesia adjunct.
Definition
ChEBI: A piperidinecarboxylate ester that is the ethyl ester of isonipecotic acid in which the hydrogen alpha- to the carboxyl group is substituted by a phenyl group, and the hydrogen attached to the nitrogen is substituted by a 2-(4-aminophenyl) thyl group.
Manufacturing Process
A mixture of 7.8 grams (0.05 mol) of β-(p-aminophenyl)ethyl chloride
hydrochloride, 12.5 grams (0.025 mol) of 4-phenyl-4-carboethoxypiperidine
carbonate, 10.5 grams (0.125 mol) sodium bicarbonate, and 100 cc of
anhydrous ethanol are mixed, stirred and heated under reflux for a period of
approximately 40 hours and then concentrated in vacuum to dryness. The
residual material is triturated with 50 cc of water, decanted, washed by
decantation with an additional 50 cc of water, and then dried in vacuum to
give N-[β-(p-aminophenyl)ethyl]-4-phenyl-4-carboethoxypiperidine.
The N-[β-(p-aminophenyl)ethyl]-4-phenyl-4-carboethoxypiperidine is dissolved
in 50 cc of hot anhydrous ethanol, an excess (about 20 cc) of 20% alcoholic
hydrochloric acid solution is added; upon scratching the side of the container
crystals form. One hundred cubic centimeters of ether are then added to the
mixture, the ethereal mixture is cooled, and the crystalline material which
precipitates is recovered by filtration, washed with ether, and dried to give
12.7 grams of N-[β-(p-aminophenyl)ethyl]-4-phenyl-4-carboethoxypiperidine
dihydrochloride which can be further purified by recrystallization from ethanol
or methanal to give substantially pure material; MP 275-277°C.
brand name
Leritine (Merck).
Therapeutic Function
Narcotic analgesic
Pharmacokinetics
Anileridine, a potent analgesic, is an analog of pethidine. Anileridine is useful for the relief of moderate to severe pain. It may also be used as an analgesic adjunct in general anesthesia in the same manner as meperidine to reduce the amount of anesthetic needed, to facilitate relaxation, and to reduce laryngospasm. In addition, anileridine exerts mild antihistaminic, spasmolytic and antitussive effects. Anileridine's main pharmacologic action is exerted on the CNS. Respiratory depression, when it occurs, is of shorter duration than that seen with morphine or meperidine when equipotent analgesic doses are used.
Safety Profile
Poison by ingestion,subcutaneous, intravenous, and intraperitoneal routes.When heated to decomposition it emits toxic fumes ofNOx.
Metabolism
Hepatic
Properties of Anileridine
| Melting point: | 83°C |
| Boiling point: | 486.07°C (rough estimate) |
| Density | 1.1096 (rough estimate) |
| refractive index | 1.6500 (estimate) |
| solubility | DMSO (Slightly), Ethyl Acetate (Soluble) |
| pka | 8.41±0.10(Predicted) |
| form | Solid |
Safety information for Anileridine
Computed Descriptors for Anileridine
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1