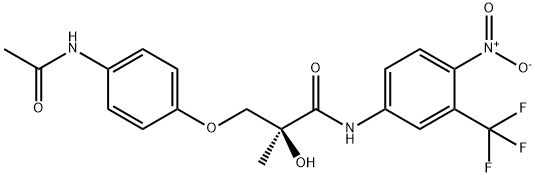
Andarine
Synonym(s):Andarine, S-4;SARM S-4
- CAS NO.:401900-40-1
- Empirical Formula: C19H18F3N3O6
- Molecular Weight: 441.36
- MDL number: MFCD09027386
- EINECS: 803-892-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-17 18:24:26
What is Andarine?
Description
Andarine (S-4) is the arylpropionamide-derived compound [S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide] first described by Yin et al. to be a selective androgen receptor modulator (SARM). Andarine (S-4) was shown by in vitro and in vivo studies to be an effective anabolic agonist, without side-effects often associated with anabolic-androgenic steroids, relating principally to substrate affinity for 5α-reductase to produce DHT in the prostate. Unfortunately the benefits that SARMs such as andarine (S-4) may provide to clinical medicine have the potential for misuse in sports where athletes and/or their handlers may seek to gain unfair advantage with the assumption that these compounds are undetectable by anti-doping laboratories. SARMs are therefore prohibited for use by the International Federation of Horseracing Authorities and the World Anti-Doping Agency (WADA).
Chemical properties
Pale Yellow Solid
The Uses of Andarine
It is a potent and tissue-selective androgen receptor modulator (SARM)
What are the applications of Application
Andarine is an androgen receptor (AR) agonist with Ki of 4 nM
Definition
ChEBI: Andarine is an anilide and a member of acetamides. It is a non-steroidal selective androgen receptor modulator.
Benefits
Andarine (S4) has a lot of great benefits and this is probably what you are most interested in.
The Andarine benefits are:
Increased muscle mass
Fat loss
Faster recovery
Body recomposition
Increased strength
Biological Activity
Andarine is a selective non-steroidal androgen receptor (AR) agonist with Ki of 4 nM, and it is tissue selective for the metabolic organ. Phase 3.
Side Effects
Andarine has gone through quite a bit of research but not as much as other SARMs such as LGD 4033 or Ostarine. From the studies so far and from the anecdotal evidence from people that use it we can tell that there are two side effects.
S4 side effects:
Suppression
Impaired vision
Synthesis
Andarine was prepared from N-[4-Nitro-3-(trifluoromethyl)phenyl]-(2R)-3-bromo-2-hydroxy-2-methylpropanamide (0.37 g, 1.0 mmol), 4-acetamidophenol (0.23 g, 1.5 mmol) K2CO3 (0.28 g, 2.0 mmol), and 10% of benzyltributylammonium chloride as a phase transfer catalyst in 20 mL of methyl ethyl ketone was heated at reflux overnight under argon. The reaction was followed by TLC, the resulting mixture was filtered through Celite, and concentrated in vacuo to dryness. Purification by flash column chromatography on silica gel (hexanes-ethyl acetate, 3:1) yielded 0.38 g (86%) (Rf=0.18 hexanes-ethyl acetate, 3:1) of the desired compound as a light yellow powder: mp 70-74° C.
in vitro
Andarine binds to androgen receptors with high affinity with Ki of 4 nM. Moreover, 10 nM Andarine stimulated the transcription adjusted by androgen receptor, up to 93%.
in vivo
Andarine has an effective metabolic activity, and stimulates the growth of the prostate, seminal vesicle, and levator ani, with ED50 is 0.43 mg/day, 0.55 mg/day, and 0.14 mg/day respectively. This effect is dose dependent. In addition, Andarine acts on FSH, which does not affect some physiological changes caused by castration, and at a dose of 0.5 mg or more per day, partially inhibiting LH production. Andarine was administered to dog intravenously at 0.1, 1, 3, and 10 mg/kg doses, then the total body clearance (CL) decreased from 7.4 mL/min/kg to 3.1 mL/min/kg, the steady-state volume (Vss) was 1.39 L/kg and the half-life was 229 minutes. In addition, Andarine was 10 mg/kg, 1 mg/kg and 0.1 mg/kg, with oral bioavailability of 38%, 62% and 91% respectively. Andarine has tissue selective pharmacological activity and is well treated with 0.5 mg/day concentration, significantly reducing prostate weight to 79.4%.
Clinical claims and research
The pharmacological activity of andarine suggest the great potential of andarine in the development of the clinically available SARMs, which offers unique therapeutic advantages over their steroidal counterparts and an exciting opportunity to differentially regulate the androgen effects in various target tissues, thus minimizing the interference to normal. Thus, andarine has promising in advanced clinical trials that aim to treat age-related maladies and to counteract symptoms of severe diseases such as sarcopenia and cancer cachexia.
However, andarine may be misused in sports where athletes and/or their handlers may seek to gain unfair advantage. Therefore, andarine as agonist is prohibited for use by the International Federation of Horseracing Authorities and the World Anti-Doping Agency.
Regulatory Status
Andarine also named SARM S-4, classified in the S1 class of the WADA prohibited list.
US: Andarine is not listed specifically in the Schedules to the US Controlled Substances Act and is not mentioned anywhere on the DEA website.
United Nations: The substance is not listed specifically on the Yellow List - List of Narcotic Drugs under International Control nor the Green List - List of Psychotropic Substances under International Control.
Canadian Status: Andarine is not currently listed in the CDSA. The substance has been reported to be used for the treatment of muscle wasting, osteoporosis and begning prostatic hyperplaysia and displays androgenic and anabolic activity1-3 . 2 3However, since andarine is not a steroid, it cannot be included uner item 23 of Schedule IV to the CDSA.
References
[1]. yin, d., et al., pharmacodynamics of selective androgen receptor modulators. j pharmacol exp ther, 2003. 304(3): p. 1334-40.
[2]. thevis, m., et al., mass spectrometric characterization of urinary metabolites of the selective androgen receptor modulator s-22 to identify potential targets for routine doping controls. rapid commun mass spectrom, 2011. 25(15): p. 2187-95.
[3]. https://www.webmd.com/vitamins/ai/ingredientmono-1586/andarine
[4]. https://en.wikipedia.org/wiki/Andarine
[5]. https://go.drugbank.com/drugs/DB07423
Properties of Andarine
| Melting point: | 70-74℃ |
| Boiling point: | 698.7±55.0 °C(Predicted) |
| Density | 1.47 |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | neat |
| pka | 12.13±0.29(Predicted) |
| form | Solid |
| color | Light Yellow |
| Water Solubility | 1.2 mg/mL in water |
| BRN | 9666695 |
Safety information for Andarine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Andarine
| InChIKey | YVXVTLGIDOACBJ-SFHVURJKSA-N |
| SMILES | C(NC1=CC=C([N+]([O-])=O)C(C(F)(F)F)=C1)(=O)[C@](O)(C)COC1=CC=C(NC(C)=O)C=C1 |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 Andarine 99% (HPLC) CAS 401900-40-1View Details
Andarine 99% (HPLC) CAS 401900-40-1View Details
401900-40-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1