Aminopterin
Synonym(s):Aminopterin;(S)-2-{4-[(2,4-Diaminopteridin-6-yl)methylamino]benzamido}pentanedioic acid;4-Aminofolic acid;4-Amino-PGA;4-Aminopteroyl-L -glutamic acid
- CAS NO.:54-62-6
- Empirical Formula: C19H20N8O5
- Molecular Weight: 440.41
- MDL number: MFCD00036692
- EINECS: 200-209-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
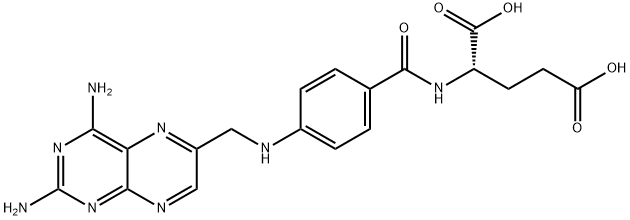
What is Aminopterin?
Description
Aminopterin is an amino derivative of folic acid, which was once used as an antineoplastic agent in the treatment of pediatric leukemia. In the 1950's its production was discontinued in favor of methotrexate, which is less potent but less toxic. Off label, aminopterin has also been used in the treatment of psoriasis. Clinicians need to be aware of the characteristic teratologic effects of aminopterin and methotrexate.
Chemical properties
Dark Yellow Solid
Chemical properties
Aminopterin is a white to yellow powder; commonly used as the dihydrate which forms clustersof yellow needles
Originator
Aminopterin,Sigma Chemical Company
The Uses of Aminopterin
Aminopterin has been used in the production of anti- neurogranin antibodies.
The Uses of Aminopterin
A folic acid antagonist. Its actively transported into cells by thefolate transporter. In the cell, its converted to a high molecular weight polyglutamate metabolite by folylpolyglutamate synthase, that, in turn, binds to dihydrofolate reductase and inhibits its activity.
The Uses of Aminopterin
antineoplastic, antirheumatic, folic acid antagonist
What are the applications of Application
Aminopterin is a dihydrofolate reductase inhibitor and folic acid antagonist
Definition
ChEBI: 4-aminofolic acid is a dicarboxylic acid. It has a role as a mutagen and an EC 1.5.1.3 (dihydrofolate reductase) inhibitor. It is functionally related to a folic acid.
Manufacturing Process
2,4,5,6-Tetraaminopyrimidine·H2SO4·H2O (75.0 g, 0.293 mole) was added to a
stirred solution of BaCl2·2H2O (71.5 g, 0.293 mole) in H2O (1.45 L) at 85-
90°C. The mixture was stirred rapidly at about 90°C for 15 min, cooled to
40°C, and filtered from BaSO4, which was washed thoroughly on a funnel withH2O. The clear, yellow filtrate was then diluted further with H2O to give a
volume of 4.35 L. This solution of the tetraaminopyrimidine·2HCl was then
added to a solution of NaOAc (4.35 L of 4 N) in which 1,3-dihydroxyacetone
(79.3 g, 0.88 mole) and cysteine·HCl·H2O (51.5 g, 0.293 mole) had just been
dissolved. The resulting solution was stirred mechanically at room
temperature while a slow stream of air was continuously passed through it for
26 hours. (Yellow-orange solid began separating after 2 hours). The mixture
was then kept in a refrigerator for 16 hours before the solid was collected,
washed successively with cold H2O, EtOH, and Et2O before it was dried to
constant weight in vacuo over P2O5 at 25°C. [The crude product mixture (47
g) was weighed in order to obtain an estimate of the volume of 48% HBr
required to form hydrobromide salts]. A mechanically stirred mixture of the
dried solid and EtOH (6.05 L) was heated to 70°C, and a solution of 48% HBr
(28 ml) in EtOH (490 ml) was added in a thin stream while the mixture was
maintained at 70-75°C. The mixture was then refluxed for about 5 min with
rapid stirring while nearly all of the solid dissolved. The hot solution was
treated with Norit and filtered through a Celite mat. The clear yellow filtrate
was kept in a refrigerator overnight while a first crop of orange colored solid
separated. The collected solid was washed with EtOH, then dried in vacuo
(56°C over P2O5) to give 17.2 g of product. The filtrate was concentrated by
evaporation (rotary evaporator) to about 2 L and then refrigerated to give a
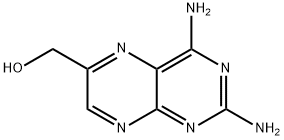
second crop of 10.2 g, which was dried as before; total yield of crude 2,4-
diamino-6-pteridinemethanol hydrobromide 27.4 g (34%). The PMR spectrum
of this material in CF3CO2D showed it to contain a barely detectable amount
of methyl-substituted 2,4-diaminopteridine·HBr.
Bromine (59.6 g, 0.373 mole) was added dropwise over a 30 min to a stirred
solution of triphenylphosphine (97.7 g, 0.373 mole) in anhydrous
dimethylacetamide (486 ml) kept at 10°C (ice bath) and protected from
atmospheric moisture. (Bromine remaining in the funnel was rinsed with 10
ml of dimethylacetamide). A smooth suspension containing finely divided,
crystalline triphenylphosphine dibromide resulted. The 2,4-diamino-6-
pteridinemethanol·HBr (25.4 g, 0.093 mole) described above was added in
one portion through a powder funnel (with the aid of 10 ml
dimethylacetamide). The ice bath was removed, and the stirred mixture was
allowed to warm to 20-25°C. After about 1 hour, complete solution had
occurred. The solution, which gradually developed a dark red color, was kept
at 20-25°C for 1 hour longer and was then chilled (ice bath) before it was
treated with EtOH (72 ml). After overnight refrigeration, the solvents were
removed by evaporation in vacuo. The dark, semisolid residue was stirred with
two 300 ml of C6H6 (to remove triphenylphosphine oxide), and each portion
was removed from the C6H6 insoluble product by decantation. The solid that
remained was dissolved with stirring in glacial AcOH (660 ml) which had been
preheated to 80°C. The mixture was kept in a bath at 80°C until solution was
complete. Tan crystalline solid separated as the dark solution was allowed to
cool. Overnight refrigeration caused the AcOH to partially freeze. When it had
thawed, the solid was collected, washed with chilled AcOH followed by Et2O,
and dried in vacuo (over P2O5 and NaOH pellets) at successive temperatures
of 25°C, 56°C, and 110°C. (The higher temperature was necessary for
complete removal of AcOH). The yield was 15.3 g (49%). (Some runs afforded
60% yield). This sample was further purified by reprecipitation from MeOH
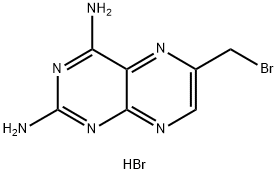
solution (Norit) by addition of Et2O followed by drying in vacuo (25°C, P2O5),
yield 13.0 g (42%) of 2,4-diamino-6-(bromomethyl)pteridine hydrobromide as A mixture of 2,4-diamino-6-(bromomethyl)pteridine hydrobromide (168 mg,
0.500 mmole) and N-(4-aminobenzoyl)-L-glutamic acid (400 mg, 1.50
mmoles) in dimethylacetamide (2 ml) was stirred at 25°C under N2 in a
stoppered flask protected from light. Solution occurred after 2 hours. After 18
hours, the orange solution was mixed with H2O (15 ml) with stirring to give a
finely divided, yellow precipitate. The mixture was centrifuged, and the
supernatant removed by decantation. The yellow solid was stirred with four 15
ml portions of H2O, each of which was removed by decantation after
centrifugation. The solid was then suspended in EtOH (15-20 ml), collected by
filtration, washed with Et2O, and dried in vacuo (25°C, P2O5) to give hydrated
N-[4-[[(2,4-diamino-6-pteridinyl)methyl]amino]benzoyl]-L-glutamic acid
(Aminopterin) hydrate (4:7) in 68% yield (160 mg). Examination by TLC
revealed one UV-absorbing spot and no fluorescence at any point.
Therapeutic Function
Antineoplastic
General Description
Clusters of yellow needles. Used as a rodenticide, medicine and rodenticide. Not registered as a rodenticide in the U.S.
Health Hazard
An antimetabolite; antagonizes the utilization of folic acid by the body. Highly toxic by ingestion.
Biochem/physiol Actions
Folic acid antagonist. Aminopterin is actively transported into cells by the folate transporter. In the cell, it is converted to a high molecular weight polyglutamate metabolite by folylpolyglutamate synthase that, in turn, binds to dihydrofolate reductase and inhibits its activity. Aminopterin-polyglutamate is degraded intracellularly by γ-glutamyl hydrolase.
Mechanism of action
Aminopterin is an amino derivative of folic acid which binds competitively to the dihydrofolate reductase enzyme to block tetrahydrofolate synthesis. Tetrahydrofolate is essential in the production of purines and pyrimadines, thus it's deficiency results in a reduction of DNA, RNA and protein synthesis.
Safety Profile
Poison by ingestion and intraperitoneal routes. Human and experimental teratogenic data. Other experimental reproductive effects. Mutation data reported. Human systemic effects by ingestion: gastrointestinal. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of NOx,.
Potential Exposure
Aminopterin is used as a medicine (as a folic acid antagonist), rodenticide, and agricultural chemical.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required
Purification Methods
Purify aminopterin by recrystallisation from H2O. It has properties similar to those of methotrexate (above). It has UV at max 244, 290 and 355nm ( 18600, 21300 and 12000) in H2O at pH 1; 260, 284 and 370nm ( 28500, 26400 and 8600) in H2O at pH 13. [Seeger et al. J Am Chem Soc 71 1753 1949, Angier & Curran J Am Chem Soc 8 1 2814 1959, Blakley The Biochemistry of Folic Acid and Related Pteridines, North-Holland Publ Co., Amsterdam, NY, pp 157-163 1969.] For small quantities, chromatograph it on DEAE cellulose with a linear gradient of ammonium bicarbonate pH 8 and increase the molarity from 0.1 to 0.4. Monitoring is by following the UV absorption of the fractions. For larger quantities, a near boiling solution of aminopterin (5g) in H2O (400mL) is slowly treated with small portions of MgO powder (~0.7g, calcined magnesia) with vigorous stirring until a small amount of MgO remained undissolved and the pH rises from 3-4 to 7-8. Charcoal (1g) is added to the hot solution and filtered immediately through a large sintered glass funnel of medium porosity and lined with a hot wet pad of Celite (~2-3 mm thick). The filtrate is cooled in ice, and the crystals of the Mg salt are collected by filtration and recrystallised from boiling H2O (200mL). The crystals are washed with EtOH and dried in vacuo. The Mg salt is redissolved in boiling H2O (200mL) and carefully acidified with vigorous agitation with AcOH (2mL). Pure aminopterin (3g) separates in fine yellow needles (dihydrate) which are easily filtered. The solid is washed with cold H2O, then Me2CO and dried in vacuo. If a trace of impurity is still present as shown by DEAE cellulose chromatography or TLC, repetition of the process will remove it; see UV above. [Loo J Med Chem 8 139 1965, Beilstein 26 IV 3831.] CARCINOGENIC.
Waste Disposal
It is inappropriate and possibly dangerous to the environment to dispose of expired or wastepharmaceuticals by flushing them down the toilet or discarding them to the trash. Household quantities of expired or waste pharmaceuticals may be mixed with wet cat litter or coffee grounds, double-bagged in plastic, discard in trash. Larger quantities shall carefully take into consideration applicable DEA, EPA, and FDA regulations. If possible return the pharmaceutical to the manufacturer for proper disposal being careful to properly label and securely package the material. Alternatively, the waste pharmaceutical shall be labeled, securely packaged and transported by a state licensed medical waste contractor to dispose by burial in a licensed hazardous or toxic waste landfill or incinerator
Properties of Aminopterin
| Melting point: | 225 °C |
| Boiling point: | 551.67°C (rough estimate) |
| alpha | 18 º (c=1% in 0.1N NaOH) |
| Density | 1.4378 (rough estimate) |
| refractive index | 1.6910 (estimate) |
| Flash point: | 87℃ |
| storage temp. | -20°C |
| solubility | 2 M NaOH: 50 mg/mL |
| form | powder |
| pka | pKa 5.5 (Uncertain) |
| color | yellow |
| Merck | 14,472 |
| BRN | 69045 |
| CAS DataBase Reference | 54-62-6(CAS DataBase Reference) |
| EPA Substance Registry System | Aminopterin (54-62-6) |
Safety information for Aminopterin
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08 |
| GHS Hazard Statements |
H300:Acute toxicity,oral H360:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Aminopterin
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran
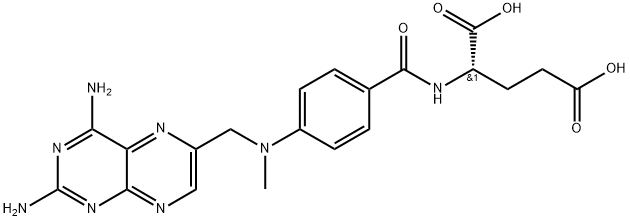
![4-[Methyl[(2-amino-4-hydroxypteridine)-6-ylmethyl]amino]benzoic acid](https://img.chemicalbook.in/CAS/GIF/5623-18-7.gif)
![L-Glutamic acid, N-[4-[[ (2-amino-1, 4-dihydro-4-oxo-6-pteridinyl)meth yl]methylamino]benzoyl]-](https://img.chemicalbook.in/CAS/GIF/2410-93-7.gif)

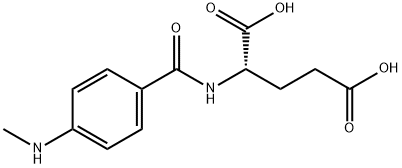
amino}benzoic acid, hemihydrochloride)](https://img.chemicalbook.in/CAS/20180808/GIF/70844-48-3.gif)
You may like
-
 Aminopterin CAS 54-62-6View Details
Aminopterin CAS 54-62-6View Details
54-62-6 -
 Aminopterin CAS 54-62-6View Details
Aminopterin CAS 54-62-6View Details
54-62-6 -
 Methotrexate Related Compound B CAS 54-62-6View Details
Methotrexate Related Compound B CAS 54-62-6View Details
54-62-6 -
 Aminopterin CAS 54-62-6View Details
Aminopterin CAS 54-62-6View Details
54-62-6 -
 Aminopterin CAS 54-62-6View Details
Aminopterin CAS 54-62-6View Details
54-62-6 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
