Aminoacylase
Synonym(s):Acylase ‘Amano’;Aminoacylase;Aminoacylase, immobilized;N-Acylamino acid amidohydrolase;Plexazym AC
- CAS NO.:9012-37-7
- Empirical Formula: C30H34Cl2N4O
- Molecular Weight: 537.52316
- MDL number: MFCD00081285
- EINECS: 232-732-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
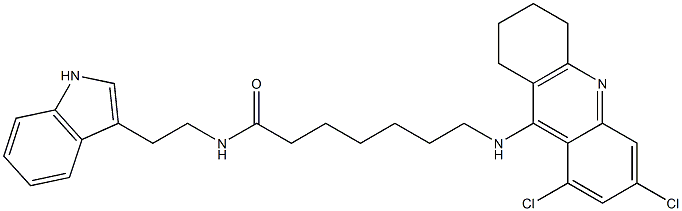
What is Aminoacylase?
Description
Aminoacylase-1 (EC 3.5.1.14) is a homodimeric zinc-binding metalloenzyme. A cytosolic enzyme with a wide range of tissue expression, it cleaves acylated L-amino acids (except L-aspartate) into L-amino acids and an acyl group. L-aspartate derivatives are cleaved by aminoacylase-2 (aspartoacylase). Aminoacylase-1 is the most abundant of the aminoacylases, a class of enzymes involved in hydrolysis of N-acetylated proteins.
The Uses of Aminoacylase
Acylase I from porcine kidney has been used to study the acylase I-catalyzed deacetylation of various S-alkyl-N-acetyl-L-cysteines and their carbon and oxygen analogues . Acylase I may be useful to catalyze N-acetyl amino acids to enantiomerically pure L-amino acids .
What are the applications of Application
Acylase I from porcine kidney has been used to study the acylase I-catalyzed deacetylation of various S-alkyl-N-acetyl-L-cysteines and their carbon and oxygen analogues . Acylase I may be useful to catalyze N-acetyl amino acids to enantiomerically pure L-amino acids.
Biological Functions
Aminoacylases (N-acyl-L-amino acid amidohydrolases; EC 3.5.1.14) are widely found in animals, plants and microorganisms. The primary function of these enzymes is to remove acyl residues from N-acetylated amino acids although they may also be capable of hydrolysing carboxylic acid amides to fatty acid anions and L-amino acids. Although the catalytic mechanism of aminoacylases has been known for decades, the physiological role of these enzymes is still poorly understood. Activities of a similar nature, however, have been found in certain carboxypeptidases, aminopeptidases and dipeptidases. It could be therefore suggested that aminoacylases have a role to play in protein/peptide turnover.
General Description
Acylase I belongs to the aminoacylase family of enzymes.
Biochem/physiol Actions
Acylase I catalyzes the deacetylation of N-acetyl-L-cysteine and S-alkyl-N-acetyl-L-cysteines. n-Butylmalonic acid is an inhibitor of acylase I. S-alkyl-N-acetyl-L-cysteines with short (C0-C3) and unbranched S-alkyl substituents have been found to be good acylase I substrates .
Enzyme inhibitor
Aminoacylase is a metallo-enzyme that needs Zinc (Zn2+) as a cofactor to function. The Zinc ions inside of aminoacylase are each coordinated to histidine, glutamate, aspartate, and water. The Zinc ion polarizes the water, facilitating its deprotonation by a nearby basic residue. The negatively charged hydroxide ion is nucleophilic and attacks the electrophilic carbonyl carbon of the substrate's acyl group.The exact mechanism after this point is unknown, with one possibility being that the carbonyl then reforms, breaks the amide bond, and forms the two products. At some point in the mechanism, another water molecule enters and coordinates with Zinc, returning the enzyme to its original state.
The nucleophilic attack by water is the rate-limiting step of aminoacylase's catalytic mechanism. This nucleophilic attack is reversible while the subsequent steps are fast and irreversible. This reaction sequence is an example of Michaelis–Menten kinetics, allowing one to determine KM, Kcat, Vmax, turnover number, and substrate specificity through classic Michaelis-Menten enzyme experiments. The second and third forward steps cause the formation and release of the reaction's products.
Properties of Aminoacylase
| storage temp. | 2-8°C |
| form | salt-free, lyophilized powder |
| color | yellow-brown |
| EPA Substance Registry System | Aminoacylase (9012-37-7) |
Safety information for Aminoacylase
| Signal word | Danger |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H334:Sensitisation, respiratory H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aminoacylase
Aminoacylase manufacturer
Anthem Biosciences Pvt Ltd
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 9012-37-7 Acylase I 99%View Details
9012-37-7 Acylase I 99%View Details
9012-37-7 -
 Acylase from Aspergillus genus CAS 9012-37-7View Details
Acylase from Aspergillus genus CAS 9012-37-7View Details
9012-37-7 -
 Acylase I from Aspergillus melleus CAS 9012-37-7View Details
Acylase I from Aspergillus melleus CAS 9012-37-7View Details
9012-37-7 -
 Acylase 1 (ACY1) ex. 0cine Kidney CAS 9012-37-7View Details
Acylase 1 (ACY1) ex. 0cine Kidney CAS 9012-37-7View Details
9012-37-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1