Ametryn
Synonym(s):2-Ethylamino-4-isopropylamino-6-methylthio-1,3,5-triazine
- CAS NO.:834-12-8
- Empirical Formula: C9H17N5S
- Molecular Weight: 227.33
- MDL number: MFCD00055398
- EINECS: 212-634-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
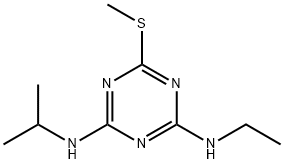
What is Ametryn?
Chemical properties
White, crystalline powder. Slightly soluble in water; soluble in organic solvents.
Chemical properties
Ametryn is a colorless powder
The Uses of Ametryn
Ametryn is a methylthiotriazine based herbicide which inhibits photosynthesis and other enzymatic processes. Ametryn is used to control broadleaf weeds and annual grasses in pineapple, sugarcane and b ananas.
The Uses of Ametryn
Pre-emergence and post-emergence herbicides.
The Uses of Ametryn
Herbicide used to control broad-leaved and grass weeds in corn, sugarcane, certain citrus subtropical fruits (bananas, pineapple) and in noncropland. Preharvest and postharvest desiccant used in potatoes to control both crop and weeds
Definition
ChEBI: A methylthio-1,3,5-triazine that is 2-(methylsulfanyl)-1,3,5-triazine substituted by an ethylamino and an isopropylamino group at positions 4 and 6 respectively.
Production Methods
Ametryn is a colorless crystal synthesized by reacting atrazine with methyl mercaptan in the presence of an equivalent of NaOH or by reacting 2-mercapto-4-ethylamino-6-isopropylamino- 1,3,5-triazine with a methylating agent in the presence of NaOH. It is stable in slightly acidic or alkaline media, but is hydrolyzed to the inactive 6-hydroxy derivative in strong acidic or basic media.
General Description
Crystals. Melting point 190-192°F (88-89°C). Used as a herbicide.
Reactivity Profile
An amine, organosulfide. Organosulfides are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides.
Hazard
Toxic by ingestion.
Health Hazard
Moderately toxic by ingestion; human toxic-ity data not available; a mild irritant to skinand eye.
LD50 oral (rat): 508 mg/kg
LD50 oral (mouse): 965 mg/kg
LD50 skin (rabbit): >5000 mg/kg.
Flammability and Explosibility
Non flammable
Agricultural Uses
Herbicide: Ametryn is a herbicide which inhibits photosynthesis and other enzymatic processes. It is used to control broadleaf weeds and annual grasses in pineapple, sugarcane and bananas. Uses are being supported in the U.S. only for the following agricultural crops: field corn, popcorn, sugarcane, and pineapple (EPA, 2005). Used in premixes with atrazine, diuron, simazine, and terbutryn. Not approved for use in the EU.
Trade name
AMESIP®; AMERTREX®; AMETREX; AMETRON SC®; AMETRYNE TECHNICAL®; AMIGAN®; AMULEX; CRISATRINE®; CRISATRINA®; DORUPLANT®; EVIK®; G-34162®; GESAPAX®; HERBIPAK®; KRISMAT®; OXON PRIMATOL Z 80®; SANCOPAX®; TRINATOX-D®
Potential Exposure
Ametryn, a triazine and an organosulfide, amine compound A potential danger to those involved in the manufacture, formulation and application of this selective herbicide.
Carcinogenicity
No carcinogenic response was observed at the highest dose tested in lifetime studies, 2000 ppm, for both rats and mice.
Environmental Fate
Biological. Cook and Hütter (1982) reported that bacterial cultures were capable of
degrading ametryne forming the corresponding hydroxy derivative (hydroxyametryne)
Soil. Although no products were reported, the half-life in soil is 70–120 days (Worthing
and Hance, 1991)
Groundwater. According to the U.S. EPA (1986) ametryn has a high potential to leach
to groundwater
Chemical/Physical. Hydrolyzes to the 6-hydroxy analog, especially in the presence of
Photolytic. The dye-sensitized photodecomposition of ametryn was studied in aqueous,
aerated solutions (Rejto et al., 1983). When an aqueous ametryn solution was irradiated
in sunlight for several hours, 2-(methylthio)-4-(isopropylamino)-6-amino-
Shipping
UN2763 Triazine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Incompatibilities
Triazines are incompatible with nitric acid. Amines are chemical bases, they neutralize acids to form salts plus water with an exothermic reaction. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents such as hydrides, nitrides, alkali metals, and sulfides.
Waste Disposal
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers.
Properties of Ametryn
| Melting point: | 84-85°C |
| Boiling point: | 310.44°C (rough estimate) |
| Density | 1.1232 (rough estimate) |
| vapor pressure | 0Pa at 20℃ |
| refractive index | 1.5500 (estimate) |
| Flash point: | 11 °C |
| storage temp. | Keep in dark place,Sealed in dry,2-8°C |
| solubility | Chloroform (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 3.71±0.41(Predicted) |
| form | Crystalline |
| color | White to off-white |
| Water Solubility | 209mg/L(25 ºC) |
| Merck | 13,391 |
| BRN | 613099 |
| CAS DataBase Reference | 834-12-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 1,3,5-Triazine-2,4-diamine, N-ethyl-N'-(1-methylethyl)-6-(methylthio)-(834-12-8) |
| EPA Substance Registry System | Ametryn (834-12-8) |
Safety information for Ametryn
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Ametryn
| InChIKey | RQVYBGPQFYCBGX-UHFFFAOYSA-N |
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Ametryn 98% (HPLC) CAS 834-12-8View Details
Ametryn 98% (HPLC) CAS 834-12-8View Details
834-12-8 -
 Ametryn CAS 834-12-8View Details
Ametryn CAS 834-12-8View Details
834-12-8 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8