Aluminum borohydride
- CAS NO.:16962-07-5
- Empirical Formula: AlB3H12
- Molecular Weight: 71.509818
- MDL number: MFCD01740733
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
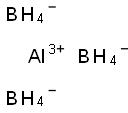
What is Aluminum borohydride?
Chemical properties
Volatile, pyrophoric liquid.
The Uses of Aluminum borohydride
Reducing agent; preparation of borohydrides of heavy metals; fuel for jet engines and rockets.
General Description
A clear, colorless liquid. Slightly denser than water. Contact causes burns to skin, eyes, and mucous membranes. Toxic by ingestion, inhalation and skin absorption. Used to make other chemicals.
Air & Water Reactions
Pyrophoric and explodes in oxygen, but only in the the presence of traces of moisture. Reacts vigorously with water, liberating hydrogen [Merck 11th ed. 1989]. Will flame when in contact with water [Ellern 1968 p. 45].
Reactivity Profile
A powerful reducing agent. Reacts rapidly and dangerously with oxygen and with other oxidizing agents, even weak ones. Incompatible with acids, alcohols, amines, and aldehydes. The borohydride reacts with alkenes and in the presence of oxygen, combustion is initiated even in the absence of moisture. Butene explodes after an induction period, while butadiene explodes immediately. Reaction with dimethylaminoborane or dimethylaminodiborane gives an oily substance which ignites in air and reacts violently with water, [J. Amer. Chem. Soc., 1951, 73, 953].
Hazard
Ignites spontaneously in air, reacts violently with water.
Health Hazard
Fire will produce irritating, corrosive and/or toxic gases. Inhalation of decomposition products may cause severe injury or death. Contact with substance may cause severe burns to skin and eyes. Runoff from fire control may cause pollution.
Fire Hazard
Flammable/combustible material. May ignite on contact with moist air or moisture. May burn rapidly with flare-burning effect. Some react vigorously or explosively on contact with water. Some may decompose explosively when heated or involved in a fire. May re-ignite after fire is extinguished. Runoff may create fire or explosion hazard. Containers may explode when heated.
Safety Profile
A poison. Spontaneously flammable in air. Explodes in oxygen with traces of water. Incompatible with alkenes and water. See also ALUMINUM COMPOUNDS and BORON COMPOUNDS.
Properties of Aluminum borohydride
| Melting point: | -64.5° |
| Boiling point: | bp 44.5°; bp119 0° |
| solubility | reacts with H2O |
| form | flammable liquid |
| color | flammable liquid |
| Water Solubility | reacts vigorously with H2O and HCl evolving H2 [MER06] |
| CAS DataBase Reference | 16962-07-5 |
| EPA Substance Registry System | Borate(1-), tetrahydro-, aluminum (3:1) (16962-07-5) |
Safety information for Aluminum borohydride
Computed Descriptors for Aluminum borohydride
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1