ALPHAPRODINE/ANADOL HYDROCHLORIDE
- CAS NO.:49638-24-6
- Empirical Formula: C16H23NO2.ClH
- Molecular Weight: 297.824
- Update Date: 2023-05-09 09:30:30
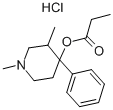
What is ALPHAPRODINE/ANADOL HYDROCHLORIDE?
Originator
Nisentil ,Roche ,US ,1949
Manufacturing Process
In a round-bottom flask provided with stirrer, dropping funnel, condenser and
a gas outlet for keeping the system under nitrogen, 200 cc of dry ether is
placed and 4.6 grams of lithium cut into thin strips is added. 52 grams of
bromobenzene in 50 cc of dry ether are added dropwise and after addition,
the mixture is refluxed for 2 hours. This procedure results in the formation of
phenyl-lithium. Other aryl-lithium compounds can be prepared in a similar
manner by reacting lithium metal or a lithium compound capable of
transferring lithium and a compound having an exchangeable halogen group
as, for example, bromonaphthalene.
The solution of phenyl-lithium is cooled to -20°C and to this a solution of 12.7
grams of 1,3-dimethyl-4-piperidone, prepared according to the method of
Howton, J. Org. Chem. 10, 277 (1945), in ether is added dropwise with
stirring. After the addition, the stirring is continued for a further 2 hours at -
20°C. The lithium complex, 1,3-dimethyl-4-phenyl-4-oxylithium piperidine,
which forms is soluble in the ether and can be recovered there from. To
prepare the piperidinol, the lithium complex, while in the reaction mixture is
decomposed by the addition of an ice and hydrochloric acid mixture. The
acidified layer is separated, basified and extracted with ether. After drying the
ether solution and removing the solvent, the residue on distillation in vacuum
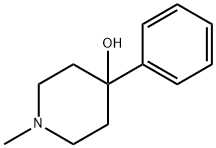
distills chiefly at 155°C/10 mm, yielding the product, 1,3-dimethyl-4-phenyl-
4-hydroxypiperidine, which, on crystallization from n-hexane melts at 102°C.
On treatment with propionic anhydride catalyzed with a trace of sulfuric acid,1,3-dimethyl-4-propionoxy-4-phenylpiperidine is attained. The latter
compound can be converted into the hydrochloride salt by reaction with
hydrogen chloride. This salt after crystallization from acetone has a melting
point of 209°C.
Therapeutic Function
Narcotic analgesic
Safety information for ALPHAPRODINE/ANADOL HYDROCHLORIDE
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
