ALPHA-ZEARALENOL
Synonym(s):2,4-Dihydroxy-6-(6α,10-dihydroxy-trans-1-undecenyl)benzoic acid μ-lactone;2,4-Dihydroxy-6-(6α,10-dihydroxy-trans-1-undecenyl)benzoic acid μ-lactone solution
- CAS NO.:36455-72-8
- Empirical Formula: C18H24O5
- Molecular Weight: 320.38
- MDL number: MFCD16657008
- EINECS: 828-728-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-07 19:09:50
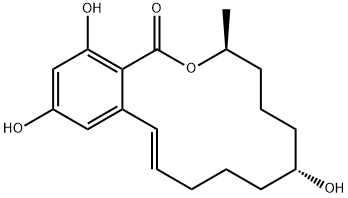
What is ALPHA-ZEARALENOL?
Description
α-Zearalenol is a major hepatic metabolite of zearalenone , a mycotoxin produced by fungi in food and animal feeds. It is a less potent agonist of estrogen receptors than the parent compound. However, α-zearalenol has pronounced effects on uterotropic activity, sperm motility, and preimplantation embryonic development.
Chemical properties
Off-White Solid
The Uses of ALPHA-ZEARALENOL
The major metabolites of Zearalenone
The Uses of ALPHA-ZEARALENOL
α-Zearalenol is a mycotoxin produced by several species of Fusarium. α-Zearalenol exhibits pronounced estrogenic activity, being 3-fold more active than zearalenone. Contamination of grains, notably maize, by Fusarium species gives rise to high levels of zearalenol and is regarded as an important food quality issue for both humans and animal health.
The Uses of ALPHA-ZEARALENOL
α- Zearalenol may be used as an analytical reference standard for the determination of the analyte in traditional medicinal herbs, human and animal urine by various chromatography techniques.
What are the applications of Application
α-Zearalenol is an estrogenic synthetic derivative of the Zeralenone mycotoxin
Definition
ChEBI: Alpha-Zearalenol is a macrolide.
Biochem/physiol Actions
α-Zearalenol (α-ZOL) is a catabolite of zearalenone, synthesized majorly in granulosa cells, intestine and liver in pigs. It has estrogenic functionality. α-ZOL inhibits sperm motility in horse possibly by eliciting genotoxic effect. α-ZOL displays higher affinity towards estrogenic receptors.
in vitro
the binding characteristic of α-zea was less potent with estrogen receptors than the parent compound [2]. α-zearalenol showed pronounced effects on uterotropic activity [3]. α-zea inhibited normal sperm motility, but stimulated hyperactive motility in the remaining motile cells and simultaneously induced the acrosome reaction [4].
References
[1]. zinedine a, soriano j m, molto j c, et al. review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: an oestrogenic mycotoxin[j]. food and chemical toxicology, 2007, 45(1): 1-18.
[2]. kiang d t, kennedy b j, pathre s v, et al. binding characteristics of zearalenone analogs to estrogen receptors[j]. cancer research, 1978, 38(11 part 1): 3611-3615.
[3]. mirocha c j, pathre s v, behrens j, et al. uterotropic activity of cis and trans isomers of zearalenone and zearalenol[j]. applied and environmental microbiology, 1978, 35(5): 986-987.
[4]. filannino a, stout t a e, gadella b m, et al. dose-response effects of estrogenic mycotoxins (zearalenone, alpha-and beta-zearalenol) on motility, hyperactivation and the acrosome reaction of stallion sperm[j]. reproductive biology and endocrinology, 2011, 9(1): 134.
Properties of ALPHA-ZEARALENOL
| Melting point: | 158-161°C |
| Boiling point: | 599.0±50.0 °C(Predicted) |
| Density | 1.174±0.06 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | -20°C |
| solubility | ≤20mg/ml in ethanol;30mg/ml in DMSO;30mg/ml in dimethyl formamide |
| form | crystalline solid |
| pka | 7.61±0.60(Predicted) |
| color | White to off-white |
| Stability: | Hygroscopic |
Safety information for ALPHA-ZEARALENOL
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Health Hazard GHS08 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H351:Carcinogenicity H371:Specific target organ toxicity, single exposure H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ALPHA-ZEARALENOL
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 α-Zearalenol solution CAS 36455-72-8View Details
α-Zearalenol solution CAS 36455-72-8View Details
36455-72-8 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
