Allopurinol
Synonym(s):1H-Pyrazolo(3,4-d)pyrimidin-4-ol;4-Hydroxypyrazolo(3,4-d)pyrimidine;4-Hydroxypyrazolo[3,4-d]pyrimidine;Allopurinol;HPP
- CAS NO.:315-30-0
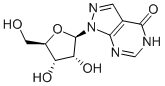
- Empirical Formula: C5H4N4O
- Molecular Weight: 136.11
- MDL number: MFCD00599413
- EINECS: 206-250-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-02-26 16:11:14

What is Allopurinol?
Absorption
This drug is about 90% absorbed from the gastrointestinal tract. Peak plasma levels normally occur at 1.5 hours and 4.5 hours post-dose for allopurinol and oxipurinol respectively. Following one oral dose of 300 mg of allopurinol, maximum plasma levels of about 3 mcg/mL of allopurinol and 6.5 mcg/mL of oxipurinol were measured .
Toxicity
Oral TDLO (rat): 10 mg/kg; Oral LD50 (mouse): 78 mg/kg; Oral TDLO (mouse): 100 mg/kg
Use in pregnancy
Reproductive studies have been completed using rats and rabbit models at doses up to twenty times the normal human dose ( about 5 mg/kg per day), and it was concluded that fertility was not impaired and there was no fetal harm. There is a published report of a study in pregnant mice administered 50 or 100 mg/kg allopurinol intraperitoneally on gestation days 10 or 13. There were increased numbers of dead fetuses in dams administered 100 mg/kg allopurinol, however, death did not occur in those given 50 mg/kg. There were higher numbers of external malformations in fetuses at both doses of allopurinol on gestation day 10 and higher numbers of skeletal malformations in fetuses at both doses on gestation day 13. Despite the above findings, there are no adequate or well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if it is absolutely required .
Use in nursing
Both allopurinol and the metabolite oxipurinol have been found in the milk of a mother who was receiving allopurinol. Since the effect of allopurinol on the nursing infant is unknown, it is advisable to exercise caution when allopurinol is taken by a nursing woman .
Mutagenicity and carcinogenicity
Cytogenic studies demonstrate that allopurinol does not induce chromosomal abnormalities in human blood cells in vitro at concentrations up to 100 g/mL and in vivo at doses up to 60 mg/day for an average duration of 40 months. Allopurinol does not form nitroso compounds (which may be carcinogenic) or affect lymphocyte transformation in vitro. Evidence suggests that allopurinol does not have deleterious effects on DNA at any stage of the cell cycle and was not found to be mutagenic. No evidence of carcinogenicity has been observed in mice treated with allopurinol for up to a 2 year period .
Description
Allopurinol is best known as the “gout medicine.” Allopurinol belongs to a class of medications called xanthine oxidase inhibitors. By inhibiting the xanthine oxidase enzyme, it decreases high concentrations of uric acid in the blood. High levels of uric acid may cause gout attacks or kidney stones. Allopurinol is used to treat gout (a type of arthritis in which uric acid, a naturally occurring substance, builds up in the joints and causes sudden attacks of redness, swelling, pain, and heat in one or more joints). Allopurinol is used to prevent gout attacks, not to treat them once they occur. Allopurinol does not need to be stopped during an acute flare. It is used to prevent or treat gout, uric acid–based kidney stones, and (formerly) chemotherapiy-induced uric acid formation in the blood.
Chemical properties
White to Off-White Solid
Originator
Zyloprim ,Burroughs-Wellcome ,US ,1966
The Uses of Allopurinol
Allopurinol does not reduce serum uric acid levels by increasing renal uric acid excretion; instead it lowers plasma urate levels by inhibiting the final steps in uric acid biosynthesis.
Uric acid in humans is formed primarily by xanthine oxidase-catalyzed oxidation of hypoxanthine and xanthine to uric acid. Allopurinol (8) and its primary metabolite, alloxanthine (9) [CAS: 2465-59-0], are inhibitors of xanthine oxidase. Inhibition of the last two steps in uric acid biosynthesis by blocking xanthine oxidase reduces the plasma concentration and urinary excretion of uric acid and increases the plasma levels and renal excretion of the more soluble oxypurine precursors. Normally, in humans the urinary purine content is almost solely uric acid; treatment with allopurinol results in the urinary excretion of hypoxanthine, xanthine, and uric acid, each with its independent solubility. Lowering the uric acid concentration in plasma below its limit of solubility facilitates the dissolution of uric acid deposits. The effectiveness of allopurinol in the treatment of gout and hyperuricemia that results from hematogical disorders and antineoplastic therapy has been demonstrated.
The Uses of Allopurinol
Xanthine oxidase inhibitor; decreases uric acid production. Used in treatment of hyperuricemia and chronic gout. Antiurolithic
The Uses of Allopurinol
antihyperuricemia, antigout, antiurolithic
Indications
Allopurinol is indicated in :
1) the management of patients with signs and symptoms of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid lithiasis, and/or nephropathy).
2) the management of patients with leukemia, lymphoma and malignancies who are receiving cancer therapy which causes elevations of serum and urinary uric acid levels. Treatment with allopurinol should be discontinued when the potential for overproduction of uric acid is no longer present.
3) the management of patients with recurrent calcium oxalate calculi whose daily uric acid excretion exceeds 800 mg/day in male patients and 750 mg/day in female patients. Therapy in such patients should be carefully assessed initially and reassessed periodically to determine in each case that treatment is beneficial and that the benefits outweigh the risks.
What are the applications of Application
Allopurinol is an anti-urolithic Xanthine oxidase inhibitor
Background
Gout is a disease that occurs by the deposition of monosodium urate crystals (MSU) in body tissues, especially around joints . This disease has been well-documented in historical medical records and appears in the biographies of several prominent, historically recognized individuals .
Allopurinol is a xanthine oxidase enzyme inhibitor that is considered to be one of the most effective drugs used to decrease urate levels and is frequently used in the treatment of chronic gout . It was initially approved by the FDA in 1966 and is now formulated by several manufacturers .
Indications
Allopurinol (Zyloprim) is the drug of choice in the treatment of chronic tophaceous gout and is especially useful in patients whose treatment is complicated by renal insufficiency.
Definition
ChEBI: Allopurinol is a bicyclic structure comprising a pyrazole ring fused to a hydroxy-substituted pyrimidine ring. It has a role as a radical scavenger, a gout suppressant, an antimetabolite and an EC 1.17.3.2 (xanthine oxidase) inhibitor. It is an organic heterobicyclic compound and a nucleobase analogue. It derives from a hydride of a 1H-pyrazolo[4,3-d]pyrimidine.
Manufacturing Process
3-Morpholino-2-cyanoacrylamide: A stirred mixture of cyanoacetamide (63 g),
triethylorthoformate (134 g), morpholine (82.5 g) and acetonitrile (37.5 ml)
was heated under reflux for 4 hours. The initial reflux temperature was 117°C
and the final reflux temperature was 82°C.
At the end of the reflux period the mixture was cooled to 30°C and the heavy crystalline precipitate was collected and washed with 2 x 75 ml of ethanol.
The product was dried in vacuum at 30°C. Wt = 111 g. Yield = 82%, MP 173-
175°C.
3-Aminopyrazole-4-carbxamide hemisulfate: To water (253 ml) at 60°C was
added 3-morpholino-2-cyanoacrylamide (63.4 g) and 85% technical hydrazine
hydrate (22.7 g). The mixture was rapidly heated to 95°C and the
temperature was maintained at >90°C for 20 minutes. The mixture was then
cooled to 60°C and the pH carefully adjusted to 1.5 by the addition of a
mixture of sulfuric acid (45.7 g) and ice. The acidified reaction was cooled to
5°C and the crystalline product collected and washed with cold water (2 x 100
ml) and acetone (2 x 50 ml). The product was dried in vacuum at 80°C. Wt
=5.8 g. Yield =95%, MP 237-239°C.
4-Hydroxypyrazolo[3,4-d]pyrimidine: A suspension of 3-aminopyrazole-4-
carboxamide hemisulfate (113 g) in formamide (325 g) was stirred and
heated to 145°C. The reaction was held at 145°C for 5 hours. The reaction
was then cooled to 30°C and the product collected and washed with
formamide (2 x 50 ml), water (2 x 150 ml) and acetone (2 x 100 ml). Wt of
crude product = 79 g. The crude product was recrystallized by dissolution in a
solution made from sodium hydroxide (25 g) in water (1,200 ml) with
treatment at 25°C with charcoal (8 g), followed by reprecipitation by the
addition of concentrated hydrochloric acid to pH 5. The product was collected
and washed with cold water (2 x 300 ml), acetone (2 x 200 ml) and dried in
vacuum at 60°C. Wt = 70 g. Yield = 80%.
brand name
Lopurin (Abbott); Lopurin (BASF); Zyloprim (Promethus).
Therapeutic Function
Xanthine oxidase inhibitor, Gout therapy
General Description
Odorless tasteless white microcrystalline powder.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Allopurinol is an aminoalcohol. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Allopurinol darkens above 572° F, and at an indefinite high temperature, Allopurinol chars and decomposes. At 221° F, maximum stability occurs at pH 3.1- 3.4. Allopurinol decomposes in acidic and basic solutions.
Fire Hazard
Flash point data for Allopurinol are not available; however, Allopurinol is probably combustible.
Biochem/physiol Actions
Inhibitor of xanthine oxidase and de novo pyrimidine biosynthesis. A classical agent in treatment of hyperuricemia and gout.
Mechanism of action
Allopurinol, in contrast to the uricosuric drugs, reduces
serum urate levels through a competitive inhibition of
uric acid synthesis rather than by impairing renal urate
reabsorption. This action is accomplished by inhibiting
xanthine oxidase, the enzyme involved in the metabolism
of hypoxanthine and xanthine to uric acid. After
enzyme inhibition, the urinary and blood concentrations
of uric acid are greatly reduced and there is a simultaneous
increase in the excretion of the more soluble
uric acid precursors, xanthine and hypoxanthine.
Allopurinol itself is metabolized by xanthine oxidase
to form the active metabolite oxypurinol, which
tends to accumulate after chronic administration of the
parent drug.This phenomenon contributes to the therapeutic
effectiveness of allopurinol in long-term use.
Oxypurinol is probably responsible for the antigout effects
of allopurinol. Oxypurinol itself is not administered
because it is not well absorbed orally.
Pharmacokinetics
Allopurinol was synthesized in 1956 as part of a study of purine antagonists. It is well absorbed on oral administration, with peak plasma concentrations appearing within 1 hour. Decreases of uric acid can be observed within 24 to 48 hours. Excretion of allopurinol and its metabolite occurs primarily in the urine, with approximately 20% of a dose being excreted in the feces.
Clinical Use
Allopurinol is especially indicated in the treatment of
chronic tophaceous gout, since patients receiving it show
a pronounced decrease in their serum and urinary uric
acid levels. Because it does not depend on renal mechanisms
for its efficacy, allopurinol is particularly beneficial
for patients who already have developed renal uric acid
stones, patients with excessively high urate excretion
(e.g., above 1,200 mg in 24 hours), patients with a variety
of blood disorders (e.g., leukemia, polycythemia vera),
patients with excessive tophus deposition, and patients
who fail to respond well to the uricosuric drugs.
Allopurinol also inhibits reperfusion injury. This injury
occurs when organs that either have been transplanted
or have had their usual blood perfusion blocked
are reperfused with blood or an appropriate buffer solution.
The cause of this injury is local formation of free
radicals, such as the superoxide anion, the hydroxyl free
radical, or peroxynitrite. These substances are strong
oxidants and are quite damaging to tissues.
Side Effects
Common toxicities associated with allopurinol administration include a variety of skin rashes, gastrointestinal upset, hepatotoxicity, and fever. These reactions are often sufficiently severe to dictate termination of drug therapy. It is advised that therapy not be initiated during an acute attack of gouty arthritis. As with the uricosuric drugs, therapy with allopurinol should be accompanied both by a sufficient increase in fluid intake to ensure water diuresis and by alkalinization of the urine. Prophylactic use of colchicine also helps to prevent acute attacks of gout that may be brought on during the initial period of allopurinol ingestion.
Safety Profile
Human poison by ingestion. Poison experimentally by intraperitoneal and subcutaneous routes. An experimental teratogen. Human systemic effects by ingestion: blood leukopenia, dermatitis, jaundice, muscle weakness, thrombocytopenia. When heated to decomposition it emits toxic fumes of NOx. An FDA proprietary drug used as a xanthine oxidase inhibitor.
Veterinary Drugs and Treatments
The principle veterinary uses for allopurinol are for the prophylactic
treatment of recurrent uric acid uroliths and hyperuricosuric calcium
oxalate uroliths in small animals. It has also been used in an
attempt to treat gout in pet birds and reptiles.
Allopurinol has been recommended as an alternative treatment
for canine Leishmaniasis. Although
it appears to have clinical efficacy,
it does not apparently clear the parasite in most dogs at usual dosages.
Allopurinol may also be useful for American Trypanosomiasis.
Drug interactions
Potentially hazardous interactions with other drugs
ACE inhibitors: increased risk of toxicity with
captopril.
Antivirals: concentration of didanosine increased -
avoid.
Ciclosporin: isolated reports of raised ciclosporin
levels (risk of nephrotoxicity).
Cytotoxics: effects of azathioprine and
mercaptopurine enhanced with increased toxicity;
avoid with capecitabine and ideally azathioprine.
Development
Roland K. Robins at New Mexico Highlands University (Las Vegas) and P. Schmidt and J. Druey at CIBA (Basel, Switzerland) independently reported allopurinol syntheses in 1956. At the time, purine derivatives were known to act as antagonists of some biological processes. Robins believed that derivatives of purine’s isomer pyrazolo [3,4-d]pyrimidine also might be biologically active. He synthesized 15 compounds, including allopurinol. He was looking specifically for new antitumor agents.
Schmidt and Druey also prepared allopurinol and other pyrazolo [3,4-d]pyrimidines in search of new pharmaceuticals for CIBA. In subsequent years, numerous investigators established allopurinol’s medicinal properties. The Food and Drug Administration approved it in the United States in 1966.
Allopurinol also has theoretical interest. In 1996, Begoa Hernández, Francisco J. Luque and Modesto Orozco at the University of Barcelona used computational methods to evaluate the relative stabilities of tautomers of allopurinol and its isomer hypoxanthine.
To avoid having to use a large number of expensive calculations (remember, this was 1996), the authors first used a low-level method to identify the seven most stable forms of both isomers. They then used ab initio quantum mechanical methods to determine the most stable tautomer of each molecule in the gas phase and the aqueous solution.
Mechanism of action
Allopurinol decreases the production of uric acid by stopping the biochemical reactions that precede its formation. Specifically, it works by inhibiting xanthine oxidase (XO), the enzyme responsible for converting hypoxanthine to uric acid, deposited as crystals in the joints of gout sufferers. Hypoxanthine is a metabolite of, and a possible precursor to, adenosine. This process decreases urate and relieves gout symptoms, which may include painful tophi, joint pain, inflammation, redness, decreased range of motion, and swelling.
Metabolism
Allopurinol is rapidly metabolized to the corresponding xanthine analog, oxipurinol (alloxanthine), which is also an inhibitor of xanthine oxidase enzyme . Both allopurinol and oxypurinol inhibit the action of this enzyme. Allopurinol and oxypurinol are also converted by the purine salvage pathway to their respective ribonucleotides. The effect of these ribonucleotides related to the hypouricemic action of allopurinol in humans is not fully elucidated to this date. These metabolites may act to inhibit de novo purine biosynthesis by inhibiting the enzyme, amidophosphoribosyltransferase. The ribonucleotides have not been found to be incorporated in DNA .
Metabolism
Allopurinol is rapidly metabolized via oxidation and the formation of numerous ribonucleoside derivatives. The major oxidation metabolite, alloxanthine or oxypurinol, has a much longer half-life (18–30 hours versus 2–3 hours) than the parent drug and is an effective, although less potent, inhibitor of xanthine oxidase. The longer plasma half-life of alloxanthine results in an accumulation in the body during chronic administration, thus contributing significantly to the overall therapeutic effects of allopurinol.
Precautions
Since allopurinol is metabolized by the hepatic microsomaldrug-metabolizing enzymes, coadministration ofdrugs also metabolized by this system should be donewith caution. Because allopurinol inhibits the oxidationof mercaptopurine and azathioprine, their individualadministered doses must be decreased by as much as75% when they are given together with allopurinol.Allopurinol may also increase the toxicity of other cytotoxicdrugs (e.g., vidarabine). The actions of allopurinolare not antagonized by the coadministration of salicylates.
Properties of Allopurinol
| Melting point: | >300 °C (lit.) |
| Boiling point: | 250.36°C (rough estimate) |
| Density | 1.4295 (rough estimate) |
| refractive index | 1.8500 (estimate) |
| storage temp. | 15-25°C |
| solubility | 1 M NaOH: soluble50mg/mL, clear to very slightly hazy, colorless to faintly yellow |
| appearance | white crystals or powder |
| pka | 10.2(at 25℃) |
| form | Powder |
| color | White or almost white |
| Water Solubility | 0.35 g/L (25 ºC) |
| Merck | 14,279 |
| CAS DataBase Reference | 315-30-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Allopurinol(315-30-0) |
| EPA Substance Registry System | Allopurinol (315-30-0) |
Safety information for Allopurinol
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for Allopurinol
| InChIKey | OFCNXPDARWKPPY-UHFFFAOYSA-N |
Allopurinol manufacturer
CEFA CILINAS BIOTICS PVT LTD
New Products
2,3-Dihydro-5,6-dimethoxy-2-(4-piperidinylmethyl)-1H-inden-1-one hydrochloride 3-Pyridineacrylic acid 2-pyridineacetonitrile Methyl 4-chloropyridine-2-carboxylate hydrochloride 1-Methyl-4-piperidinone 1-Boc-4-cyanopiperidine 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-Pyridineacetonitrile, α-hydroxy- 3-Iodophenylacetic acid 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 N N' DimethylEthylenediamine Lead II Bromide Variamine Blue B Diazonium salt Ethyl Methanesulfonate N Ethylmethylamine N N N'Trimethyl ethylenediamine (R)-2-Methylpyrolidine-2-carboxylic acid (De Mepro) Boc-his(trt)-OH Sacubitril- Valsartan Ramipril Fmoc-L-Glu-OtBu Boc LeucineRelated products of tetrahydrofuran

![3-(4-OXO-1-PHENYL-4,5-DIHYDRO-1H-PYRAZOLO[3,4-D]PYRIMIDIN-6-YL)PROPANOIC ACID](https://img.chemicalbook.in/StructureFile/ChemBookStructure4/GIF/CB1330674.gif)
![6-([2-(4-CHLOROPHENYL)-2-OXOETHYL]SULFANYL)-1-PHENYL-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMIDIN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure5/GIF/CB5118306.gif)
![5-AMINO-2,6-DIMETHYL-4,5-DIHYDRO-2H-PYRAZOLO[3,4-D]PYRIMIDIN-4-ONE](https://img.chemicalbook.in/StructureFile/ChemBookStructure2/GIF/CB2744522.gif)
![5-AMINO-1-PHENYL-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMIDIN-4-ONE](https://img.chemicalbook.in/CAS/GIF/69923-95-1.gif)
![1-METHYL-1,5-DIHYDRO-4H-PYRAZOLO[3,4-D]PYRIMIDIN-4-ONE](https://img.chemicalbook.in/CAS/GIF/5334-56-5.gif)
![3-BROMO-1H-PYRAZOLO[3,4-D]PYRIMIDIN-4-OL](https://img.chemicalbook.in/CAS/GIF/54738-73-7.gif)
You may like
-
![1H-Pyrazolo[3,4-d]pyrimidin-4-ol 98%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/b5f4aec8-0be7-4f02-a2c7-38f14f273e40.png) 1H-Pyrazolo[3,4-d]pyrimidin-4-ol 98%View Details
1H-Pyrazolo[3,4-d]pyrimidin-4-ol 98%View Details
180749-08-0 315-30-0 916980-04-6 -
 Allopurinol 98%View Details
Allopurinol 98%View Details -
![4-Hydroxy-1H-pyrazolo[3,4-d]pyrimidine CAS 315-30-0](https://img.chemicalbook.in//Content/image/CP5.jpg) 4-Hydroxy-1H-pyrazolo[3,4-d]pyrimidine CAS 315-30-0View Details
4-Hydroxy-1H-pyrazolo[3,4-d]pyrimidine CAS 315-30-0View Details
315-30-0 -
 Allopurinol 99%View Details
Allopurinol 99%View Details -
 ALLOPURINOL 99%View Details
ALLOPURINOL 99%View Details -
 Allopurinol 99%View Details
Allopurinol 99%View Details -
 Allopurinol extrapure CAS 315-30-0View Details
Allopurinol extrapure CAS 315-30-0View Details
315-30-0 -
 Allopurinol 97% CAS 315-30-0View Details
Allopurinol 97% CAS 315-30-0View Details
315-30-0
