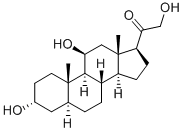
Allopregnanolone
Synonym(s):3α-Hydroxy-5α-pregnan-20-one;3α-OH-DHP;Allopregnan-3α-ol-20-one;Allopregnanolone;Brexanolone
- CAS NO.:516-54-1
- Empirical Formula: C21H34O2
- Molecular Weight: 318.49
- MDL number: MFCD00003677
- EINECS: 687-782-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
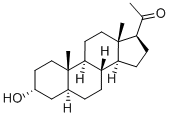
What is Allopregnanolone?
Absorption
It has been determined that brexanolone has a low oral bioavailability of approximately <5% in adults, which suggests infant exposure would also be expected to be low .
Toxicity
There is limited clinical trial experience regarding human overdosage with brexanolone . In premarketing clinical studies, two cases of accidental overdosage due to infusion pump malfunction resulted in transient loss of consciousness . Both patients regained consciousness approximately 15 minutes after discontinuation of the infusion without supportive measures . After full resolution of symptoms, both patients subsequently resumed and completed treatment . Overdosage may result in excessive sedation, including loss of consciousness, and the potential for accompanying respiratory changes .
There is no available data on brexanolone use in pregnant women to determine a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes . However, based on findings from animal studies of other drugs that enhance GABAergic inhibition, brexanolone may cause fetal harm .
Available data from a lactation study in 12 women indicate that brexanolone is transferred to breastmilk in nursing mothers . However, the relative infant dose (RID) is low, 1% to 2% of the maternal weight-adjusted dosage . Also, as brexanolone has low oral bioavailability in adults, infant exposure is expected to be low . There were no reports of effects of brexanolone on milk production . There are no data on the effects of brexanolone on a breastfed infant . Available data on the use of brexanolone during lactation does not suggest a significant risk of adverse reactions to breastfed infants from exposure to brexanolone . The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for brexanolone and any potential adverse effects on the breastfed child from brexanolone or from the underlying maternal condition .
Brexanolone was not genotoxic when tested in an in vitro microbial mutagenicity (Ames) assay, an in vitro micronucleus assay in human peripheral blood lymphocytes, and an in vivo rat bone marrow micronucleus assay .
Treatment of female and male rats with brexanolone at doses equal to and greater than 30 mg/kg/day, which is associated with 2 times the plasma levels at the maximum recommended human dose (MRHD) of 90 mcg/kg/hour, caused impairment of female and male fertility and reproduction . In female rats, brexanolone was associated with decreased mating and fertility indices, an increase in number of days to mating, prolonged/irregular estrous cycles, an increase in the number of early resorptions, and post implantation loss . Reversal of effects in females was observed following a 28-day recovery period . In male rats, brexanolone was associated with decreased mating and fertility indices, decreased conception rate, lower prostate, seminal vesicle, and epididymis weight, as well as decreased sperm numbers. Impaired female and male fertility and reproduction were not observed at 0.8 times the MRHD .
Description
Allopregnanolone is an endogenous inhibitory pregnane neurosteroid that is synthesized from progesterone . It acts as a positive allosteric modulator of GABAA receptors at nM concentrations (exhibiting the greatest potentiation at isoforms containing δ subunits) and of GABAC receptors at μM concentrations. Allopregnanolone displays effects similar to other GABAA receptor potentiators such as benzodiazepines, including potent anticonvulsant, anxiolytic, and sedative activity.
Description
Allopregnanolone, also commonly called brexanolone, is a metabolite of the sex hormone progesterone that is produced in the brains of mammals. It was first isolated from the urine of pregnant women in 1937 by Russell E. Marker, Oliver Kamm, and Ralph V. McGrew at Penn State (State College, PA) and Parke-Davis (Detroit). The following year, G. Fleischer, B. Whitman, and E. Schwenk at Schering (Bloomfield, NJ) synthesized it from the hormone pregnenolone.
Allopregnanolone modulates the γ-aminobutyric acid (GABA) receptor GABAA in the brain. In 2019, the US Food and Drug Administration approved allopregnanolone as an intravenous infusion for treating postpartum depression (PPD; see the sidebar), the first drug to be approved for this condition. Sage Therapeutics (Cambridge, MA) markets it under the trade name Zulresso.
The 60-h Zulresso infusion reduces the symptoms of PPD almost immediately. Because the patient must be closely monitored, the drug must be administered in a medical facility.
The Uses of Allopregnanolone
(3α)-Allopregnanolone acts as a GABAA receptor positive allosteric modulator. (3α)-Allopregnanolone is a metabolite of Progesterone (P755900). (3α)-Allopregnanolone is a neuroactive steroid present in the blood and also the brain.
Background
As of March 2019, brexanolone - developed and made available commercially by Sage Therapeutics Inc. as the brand name product Zulresso - is the first drug to have ever been approved by the US FDA specifically for the treatment of postpartum depression (PPD) in adult females . Since PPD, like various other types of depression, is characterized by feelings of sadness, worthlessness or guilt, cognitive impairment, and/or possibly suicidal ideation, it is considered a life-threatening condition . Studies have consequently found that PPD can genuinely have profound negative effects on the maternal-infant bond and later infant development . The development and availability of brexanolone for the treatment of PPD in adult females subsequently provides a new and promising therapy where few existed before .
In particular, the use of brexanolone in treating PPD is surrounded with promise because it acts in part as a synthetic supplement for possible deficiencies in endogenous brexanolone (allopregnanolone) in postpartum women susceptible to PPD whereas many commonly used anti-depressive medications elicit actions that may modulate the presence and activity of substances like serotonin, norepinephrine, and/or monoamine oxidase but do not mediate activities directly associated with PPD like natural fluctuations in the levels of endogenous neuroactive steroids like allopregnanolone .
And finally, although brexanolone may also be undergoing clinical trials to investigate its abilities to treat super-refractory status epilepticus, it appears that some such studies have failed to meet primary endpoints that compare success in the weaning of third-line agents and resolution of potentially life-threatening status epilepticus with brexanolone vs. placebo when added to standard-of-care .
Indications
Brexanolone is a synthetic neuroactive steroid gamma-aminobutyric acid A (GABA(a)) receptor positive modulator indicated for the treatment of postpartum depression (PPD) in adult women .
Definition
ChEBI: Brexanolone is a 3-hydroxy-5alpha-pregnan-20-one in which the hydroxy group at position 3 has alpha-configuration. It is a metabolite of the sex hormone progesterone and used for the treatment of postpartum depression in women. It has a role as a human metabolite, an antidepressant, a GABA modulator, an intravenous anaesthetic and a sedative.
Biological Activity
GABA A receptor positive allosteric modulator. Neuroactive steroid.
Pharmacokinetics
Brexanolone potentiated GABA-mediated currents from recombinant human GABA(a) receptors in mammalian cells expressing α1β2γ2 receptor subunits, α4β3δ receptor subunits, and α6β3δ receptor subunits .
Moreover, it was determined during a Phase 1 randomized, placebo and positive-controlled, double-blind, three-period crossover thorough QT study in 30 healthy adult subjects that brexanolone use did not prolong the QT interval to any clinically relevant extent when administered at 1.9-times the exposure occurring at the highest recommended infusion rate (90 mcg/kg/hour) .
Metabolism
Brexanolone is extensively metabolized by non-cytochrome (CYP) based pathways by way of three main routes - keto-reduction (via aldo-keto reductases), glucuronidation (via UDP-glucuronosyltransferases), and sulfation (via sulfotransferases) . Three predominant circulating metabolites result from such metabolic pathways and they are all pharmacologically inactive and ultimately do not contribute to the overall efficacy of the medication .
Storage
Room temperature
References
1) Belelli and Lambert (2005),?Neurosteroids: endogenous regulators of the GABA(A) receptor; Nat. Rev. Neurosci,,?6?565 2) Reddy?et al. (2010),?Neurosteroids: endogenous role in the human brain and therapeutic potentials; Prog. Brain Res.,?186?113 3) Frieder?et al. (2019),?Pharmacotherapy of Postpartum Depression: Current Approaches and Novel Drug Development; CNS Drugs,?33?265 4) Balan?et al.?(2019),?Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Proinflammatory Signaling in Macrophages and Brain; Sci. Rep.,?9?1220 5) Chang?et al.?(2019),?Neurosteroid allopregnanolone inhibits glutamate release from rat cerebrocortical nerve terminals; Synapse,?73?e22076 6) Cho?et al.?(2018),?Increased Superoxide Dismutase 2 by Allopregnanolone Ameliorates ROS-Mediated Neuronal Death in Mice with Pilocarpine-Induced Status Epilepticus; Neurochem. Res.,?43?1464
Properties of Allopregnanolone
| Melting point: | 176-178° |
| Boiling point: | 431.2±18.0 °C(Predicted) |
| alpha | D +87.7° (abs alc) |
| Density | 1.053±0.06 g/cm3(Predicted) |
| storage temp. | Store at RT |
| solubility | chloroform: 20 mg/mL, clear, colorless |
| pka | 15.12±0.70(Predicted) |
| form | White solid |
| appearance | white crystalline solid |
| color | white |
| Merck | 13,272 |
| BRN | 3211363 |
| Stability: | Stable for 2 years as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
| CAS DataBase Reference | 516-54-1(CAS DataBase Reference) |
Safety information for Allopregnanolone
Computed Descriptors for Allopregnanolone
| InChIKey | AURFZBICLPNKBZ-SYBPFIFISA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 5α-Pregnan-3α-ol-20-one CAS 516-54-1View Details
5α-Pregnan-3α-ol-20-one CAS 516-54-1View Details
516-54-1 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
