Alizarin
Synonym(s):1,2-Dihydroxyanthraquinone;Mordant Red 11
- CAS NO.:72-48-0
- Empirical Formula: C14H8O4
- Molecular Weight: 240.21
- MDL number: MFCD00001201
- EINECS: 200-782-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:47
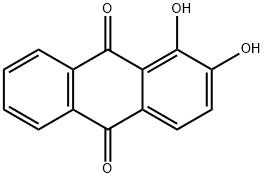
What is Alizarin?
Chemical properties
orange-red crystals or powder
History
In ancient times, alizarin was the preferred red dye. Cloth dyed with it has been found in Egyptian tombs dating 6000 years ago. The dye is found in the madder plant, a member of the Rubiaceae family. In 1944 about 35 species of this plant were known, but the use of more sophisticated analytical methods led to the detection of many more species; by 1984 the number had increased to 50. Alizarin is a mordant dye forming various colored coordination complexes with different metallic salts.
The Uses of Alizarin
Alizarin is used widely as a prominent red dye for textile facbrics. Alizarin red is now used in biochemical assay to determine quantitatively by colorimetry, the presence of calcific deposition by ce lls of the osteogenic lineage. Alzarine derivatives was evaluated as new inhibitors of the HIV-1 reverse transcriptase associated DNA polymerase and RNase H. Alizarin was also shown to inhibit prolife ration, tumor growth and suppress tumorigenesis in human osteosarcoma and breast cancer cell representative for bone metastasis.
The Uses of Alizarin
antimutagen
The Uses of Alizarin
Alizarin has a major application in the manufacture of the madder lake pigments which is commonly called as Rose madder and Alizarin crimson. It is involved in the studies of bone growth, osteoporosis, bone marrow and calcium deposits in the vascular system. It is also used as a stain in calcite and aragonite to identify calcium carbonate, and synovial fluid to asses for basic calcium phosphate crystals. It is used in colorimetric measurements for quantification of amine extraction by model food simulants from epoxy polymer.
What are the applications of Application
Alizarin is a biological stain with color index number: 58000
Definition
ChEBI: A dihydroxyanthraquinone that is anthracene-9,10-dione in which the two hydroxy groups are located at positions 1 and 2.
Definition
An important orange-red organic compound used in the dyestuffs industry to produce red lakes. It occurs naturally in the root of the plant madder and may also be synthesized from anthraquinone.
Definition
alizarin: An orange-red compound,C14H8O4. The compound is a derivativeof anthraquinone, with hydroxylgroups substituted at the 1and 2 positions. It is an importantdyestuff producing red or violetlakes with metal hydroxide.Alizarin occurs naturally as the glucosidein madder. It can be synthesizedby heating anthraquinone withsodium hydroxide.
Preparation
(a) 2 – Bromoanthraquinone and Potassium hydroxide heating; (b) 9,10-Dioxo-9,10-dihydroanthracene-2-sulfonic acid (Sodium) and Sodium hydroxide and Sodium nitrate and Sodium chlorate heating; (C) Anthracene-9,10-dione?and Sodium hydroxide and Sodium chlorate and Sodium nitrate heating (GP 186526); (d) in the presence of Sodium nitrite and Sodium hydroxide and Anthracene-9,10-dione?and Sodium nitrate heating (GP 241806245 987); (e) in the Oxidant and Sodium sulfite and the presence of lime, anthracene in a nitrocellulose derivatives and Sodium hydroxide heating (GP 292247); (f) 2 – Anthraquinonesulfonic acid in the presence of air, with the Sodium hydroxide Etanol wetting treatment (GP 287270); (g) 2-Chloroanthracene-9,10-dione Sodium chlorate in the presence of alkali fusion for (USP 1744815); (h) 2-Methylanthracene-9,10-dione in the presence of Oxidant, with Sodium hydroxide treatment (BP 293328).
Biological Activity
alizarin is an anthraquinone dye for detecting the presence of calcium salts [1].alizarin belongs to the anthraquinone group. it is a chelator for calcium and is commonly used to stain the calcifying or calcio-receptive zone of the collagenous matrix where calcium salts are being deposited. on the other hand, the alizarin complexone is used for bone staining in vivo to study bone remodeling [1].alizarin is proved to have anti-tumor efficacy. it suppresses the cell growth of the prostate cancer, breast cancer and osteosarcoma cell lines in vitro. among these, the osteosarcoma cells appear to be most sensitive. the ic50 values of alizarin against three osteosarcoma cell lines saos-2, mg-63 and u-2 os are 27.5, 29 and 69.9μg/ml, respectively. alizarin inhibits the cell growth through cell proliferation blockade rather than induction of apoptosis. it inhibits the phosphorylation of erk. in addition, alizarin is also found to induce s-phase arrest as well as a decrease of the g0/g1 and g2/m phases [1].
Synthesis
1,2-Dihydroxyanthraquinone is prepared from anthraquinone by alkali melt or from anthraquinone-2-sulfonic acid with 50 % aqueous sodium hydroxide and sodium nitrate at 190 – 200 ?C .
Purification Methods
Alizarin crystallises from glacial acetic acid or 95% EtOH. It can also be sublimed at 110o/2mm. It is an indicator with max at 452nm (pH 5.8) and 520nm (pH 7.2). [Beilstein 8 IV 3256.]
Properties and Applications
commonly known as 1,2-Dihydroxyanthracene-9,10-dione , calcium, aluminum mordanting for red to blue light red, chromium for dark blue light red, iron as dark purplish red. Claybank powder. Slightly soluble in water, but soluble in Methanol, Acetone Etanol, boiling, alkali and Cellosolve, slightly soluble in benzene, Carbon tetrachloride, insoluble in cold Etanol, Stout solvent, soluble in alkali liquor for royal purple. In concentrated sulfuric acid for yellow red, red yellow after diluted produce precipitation; In the Sodium hydroxide to purple. Used for wool fabric dyeing and used as organic pigment.
| Standard | Ironing Fastness | Light Fastness | Fulling | Persperation Fastness | Soaping | Water | |
| Alkali | Acid | ||||||
| ISO | 3-4 | 7 | 3-4 | 1 | 3 | 4 | 4 |
| AATCC | Dry 2,wet 5 | 7 | 2-3 | 4 | 4 | 5 | |
References
[1] fotia c, avnet s, granchi d, baldini n. the natural compound alizarin as an osteotropic drug for the treatment of bone tumors. j orthop res. 2012 sep;30(9):1486-92.
Properties of Alizarin
| Melting point: | 287 °C |
| Boiling point: | 430 °C |
| Density | 1.06 g/mL at 20 °C |
| refractive index | 1.5190 (estimate) |
| Flash point: | 430°C subl. |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | Solubility Virtually insoluble in water; moderately soluble in ethanol, soluble in benzene, toluene, Ixylene, pyridine, acetic acid |
| Colour Index | 58000 |
| form | Fine Powder |
| pka | 6.77(at 25℃) |
| color | Orange to orange-brown |
| PH Range | Yellow (5.5) to red (6.8);Red (10.1) to purple (12.1) |
| PH | 5.5~7.2 |
| Water Solubility | Soluble in hexane and chloroform. Slightly soluble in water. |
| λmax | 567nm, 609nm |
| Merck | 14,251 |
| BRN | 1914037 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong bases. |
| Biological Applications | Detecting microorganisms; treating dermatological conditions |
| Major Application | Plasma displays, Antireflective coatings, chemical-mechanical polishing, photoreceptors, glass coatings, paints, anticorrosion coatings, thermoplastics, wood coloring, textiles, pH sensor device, detergent, hair dyes, darkening skin, cosmetics, parasiticide, antifungal agent |
| CAS DataBase Reference | 72-48-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Alizarin(72-48-0) |
| EPA Substance Registry System | Alizarin (72-48-0) |
Safety information for Alizarin
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Alizarin
| InChIKey | RGCKGOZRHPZPFP-UHFFFAOYSA-N |
Alizarin manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran





You may like
-
 72 - 48 -0 Alizarine 98%View Details
72 - 48 -0 Alizarine 98%View Details
72 - 48 -0 -
 ALIZARIN 99%View Details
ALIZARIN 99%View Details
72-48-0 -
 Alizarine, GR CAS 72-48-0View Details
Alizarine, GR CAS 72-48-0View Details
72-48-0 -
 Alizarin 95% CAS 72-48-0View Details
Alizarin 95% CAS 72-48-0View Details
72-48-0 -
 Alizarin CAS 72-48-0View Details
Alizarin CAS 72-48-0View Details
72-48-0 -
 ALIZARINE AR CAS 72-48-0View Details
ALIZARINE AR CAS 72-48-0View Details
72-48-0 -
 Alizarin CAS 72-48-0View Details
Alizarin CAS 72-48-0View Details
72-48-0 -
 5 kg AlizarinView Details
5 kg AlizarinView Details
72-48-0
