Aliskiren
Synonym(s):(2S,4S,5S,7S)-5-Amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide
- CAS NO.:173334-57-1
- Empirical Formula: C30H53N3O6
- Molecular Weight: 551.76
- MDL number: MFCD09839018
- EINECS: 605-672-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-20 11:41:24
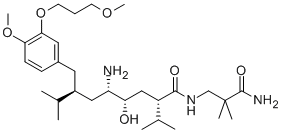
What is Aliskiren?
Absorption
Aliskiren is absorbed in the gastrointestinal tract and is poorly absorbed with a bioavailability between 2.0 and 2.5%. Peak plasma concentrations of aliskiren are achieved between 1 to 3 hours after administration. Steady-state concentrations of aliskiren are achieved within 7-8 days of regular administration.
Toxicity
The oral LD50 of aliskiren in rats is >2000 mg/kg. Overdose information is limited in the literature, however, an overdose with aliskiren is likely to result in hypotension. Supportive treatment should be initiated in the case of an overdose.
Description
Aliskiren is a first-in-class antihypertensive drug that acts by direct inhibition of renin. It is indicated for oral administration either as monotherapy or in combination with other antihypertensive agents. Renin catalyzes the cleavage of angiotensin to form angiotensin I, the first and the rate-limiting step of the RAAS. The inhibition of renin by aliskiren results in reduced levels of angiotensin I, angiotensin II, and aldosterone, all of which contribute to the antihypertensive effect. RAAS modulators are among some of the most commonly prescribed antihypertensive agents to date.The most common side effect of aliskiren is diarrhea, particularly with doses higher than 300mg daily. Unlike ACE inhibition, direct inhibition of renin does not increase bradykinin levels. Elevated bradykinin levels are thought to be responsible for the angioderma and cough that occur commonly during treatment with ACE inhibitors. .
Originator
Novartis (Switzerland)
The Uses of Aliskiren
An orally active, synthetic nonpeptide renin inhibitor. Antihypertensive
The Uses of Aliskiren
liquid crystal intermediate
Background
Aliskiren is the first drug in the renin inhibitor drug class and is used for the treatment of hypertension. It was developed by Speedel and Novartis and initially approved by the FDA in early 2007. Aliskiren has been proven to efficacious in reducing blood pressure when used alone or in conjunction with other antihypertensive agents.
Indications
Aliskiren is used for the treatment of hypertension in children above 6 years and adults. This drug may also be used in conjunction with antihypertensives such as calcium channel blockers and thiazides in products form to provide additional blood pressure control.
Definition
ChEBI: A monomethoxybenzene compound having a 3-methoxypropoxy group at the 2-position and a multi-substituted branched alkyl substituent at the 4-position.
brand name
Tekturna
General Description
Aliskiren, (2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2-methyl-propyl)-4-hydroxy-7-{[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl}-8-methyl-2-propan-2-yl-nonanamide (Tekturna), is the first renin inhibitorintroduced into the U. S. market. It was approved by theFDA in 2007 for the management of hypertension.The trade name for aliskiren in the United Kingdom isRasilez.
Pharmacokinetics
Aliskiren reduces blood pressure by inhibiting renin. This leads to a cascade of events that decreases blood pressure, lowering the risk of fatal and nonfatal cardiovascular events including stroke and myocardial infarction.
Metabolism
About 80% of the drug in plasma following oral administration is unchanged. Two major metabolites account for about 1-3% of aliskiren in the plasma. One metabolite is an O-demethylated alcohol derivative and the other is a carboxylic acid derivative. Minor oxidized and hydrolyzed metabolites may also be found in the plasma.
Properties of Aliskiren
| Melting point: | 98-99 °C |
| Boiling point: | 748.4±60.0 °C(Predicted) |
| Density | 1.067±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Store in freezer, under -20°C |
| solubility | DMSO : 100 mg/mL (181.24 mM; Need ultrasonic) |
| form | Powder |
| pka | 9.49(at 25℃) |
| color | White to light yellow |
| CAS DataBase Reference | 173334-57-1(CAS DataBase Reference) |
Safety information for Aliskiren
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aliskiren
Aliskiren manufacturer
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![Benzene, 4-[(2R)-2-(chloromethyl)-3-methylbutyl]-1-methoxy-2-(3-methoxypropoxy)-](https://img.chemicalbook.in/CAS/GIF/324763-39-5.gif)
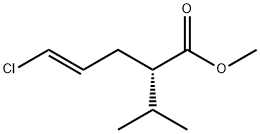
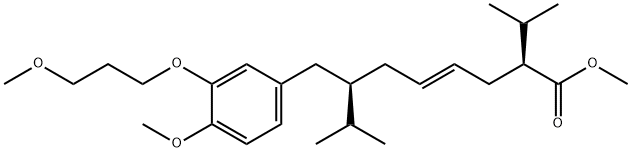
You may like
-
 173334-57-1 Aliskiren 98%View Details
173334-57-1 Aliskiren 98%View Details
173334-57-1 -
 Aliskiren 98.00% CAS 173334-57-1View Details
Aliskiren 98.00% CAS 173334-57-1View Details
173334-57-1 -
 Sodium Bifluoride CAS 173334-57-1View Details
Sodium Bifluoride CAS 173334-57-1View Details
173334-57-1 -
 Aliskiren CAS 173334-57-1View Details
Aliskiren CAS 173334-57-1View Details
173334-57-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8