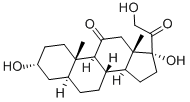
Alfaxalone
Synonym(s):3-Hydroxy-5α-pregnane-11,20-dione;Alfaxalone
- CAS NO.:23930-19-0
- Empirical Formula: C21H32O3
- Molecular Weight: 332.48
- MDL number: MFCD00083468
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-04-01 18:08:31
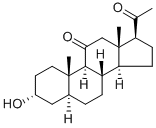
What is Alfaxalone?
Description
Alfaxalone (Alfaxan, Vetoquinol) is a neurologically active steroid compound that induces general anaesthesia. Alfaxalone exerts its action by binding to GABA receptors on the neuronal cell surface, affecting cell membrane chloride ion transport. It has negligible analgesic properties at therapeutic doses. Alfaxalone may be administered either intravenously or intramuscularly, and due to the possibility of apnoea after intravenous induction this route should be reserved for cases where intubation can be achieved[2].
Originator
Althesin ,Glaxo ,UK ,1972
The Uses of Alfaxalone
Anesthetic;nociception blocker
Definition
ChEBI: Alphaxolone is a corticosteroid hormone.
Manufacturing Process
A solution of 3α-hydroxy-5α-pregn-16-ene-11,20-dione (200 mg) in freshly distilled tetrahydrofuran (8 ml) with 5% palladium on carbon (100 ml) was hydrogenated until hydrogen uptake ceased. The mixture was filtered through a pad of kieselguhr and the tetrahydrofuran removed in vacuum to give 196 mg, MP 171°C to 172°C.
Therapeutic Function
Anesthetic component
Biological Activity
A neurosteroid anesthetic that directly activates and potentiates GABA A receptor-activated membrane current (I GABA ). Efficacy but not potency is determined by the alpha subunit of the receptor (EC 50 values are 1.4, 1.8, 2.1, 2.4 and 2.5 μ M for α 1 β 1 γ 3, α 1 β 1 γ 1, β 1 γ 1, α 2 β 1 γ 2L and α 1 β 1 γ 2L isoforms respectively).
Mechanism of action
Alfaxalone's mechanism of action is chloride ion modulation associated with interaction with the neuronal membrane GABA A receptor[1].
Side Effects
Alfaxalone is safe and effective at 12–15 mg/kg IM for routine noninvasive procedures and lasts approximately 40–45 min. The most common side effects are dose-dependent but transient hypotension (15 min), hypoventilation, and apnea. Higher doses of alfaxalone (12 mg/kg) may cause mild hypoxemia, which can be corrected by administration of supplemental oxygen via face mask. Muscle twitching occurs toward the end of immobilization and can be used as a sign of impending emergence[1].
Synthesis
It is prepared by
reduction of 3,11,20-trioxo-5-α-pregnane with
trimethyl phosphite in the presence of an iridium
catalyst. Only this reducing agent forms the
axial alcohol (3α) .
References
[1] Marini, R. and J. Haupt. “Anesthesia and Select Surgical Procedures.” The Common Marmoset in Captivity and Biomedical Research 61 1 (2019).
[2] Molly Varga BVetMed DZooMed MRCVS. “Anaesthesia and Analgesia.” Textbook of Rabbit Medicine (Second Edition) (2014): 178-202.
Properties of Alfaxalone
| Melting point: | 172-174° |
| Boiling point: | 409.59°C (rough estimate) |
| alpha | D26 +113.4° (c = 1.2 in chloroform). |
| Density | 1.0834 (rough estimate) |
| refractive index | 1.5192 (estimate) |
| storage temp. | Store at RT |
| solubility | chloroform: ≥5 mg/mL, clear, colorless |
| form | powder |
| pka | 15.05±0.70(Predicted) |
| color | white |
| InChI | InChI=1/C21H32O3/c1-12(22)16-6-7-17-15-5-4-13-10-14(23)8-9-20(13,2)19(15)18(24)11-21(16,17)3/h13-17,19,23H,4-11H2,1-3H3/t13-,14+,15-,16+,17-,19+,20-,21+/s3 |
Safety information for Alfaxalone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Alfaxalone
| InChIKey | DUHUCHOQIDJXAT-YYJIQYHHNA-N |
| SMILES | [C@]12([H])C(=O)C[C@]3(C)[C@H](CC[C@@]3([H])[C@]1([H])CC[C@@]1([H])C[C@H](O)CC[C@]21C)C(=O)C |&1:0,5,7,10,12,16,19,23,r| |
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran




You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4