AHU-377
- CAS NO.:149709-62-6
- Empirical Formula: C24H29NO5
- Molecular Weight: 411.49
- MDL number: MFCD00920862
- EINECS: 1592732-453-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is AHU-377?
Absorption
Peak plasma concentrations of sacubitril and it's metabolite, LBQ657 are reached in 0.5 hours and 2 hours respectively. Food does not clinically affect the systemic exposure of sacubitril or LBQ657. The oral bioavailability of sacubitril is >60%. It should be noted that the valsartan found in this combination is more bioavailable than other market available valsartan.
Toxicity
The most common adverse reactions (≥5%) are hypotension, hyperkalemia, cough, dizziness, and renal failure.
The Uses of AHU-377
Sacubitril is an antihypertensive drug used in combination with valsartan. The combination drug, valsartan/sacubitril, known during trials as LCZ696 and marketed under the brand name, Entresto, is a treatment for heart failure.
What are the applications of Application
AHU 377 is an inhibitor of neprilysin with IC50 value of 5 nM.
Definition
ChEBI: Sacubitril is a member of biphenyls.
Indications
Used in combination with valsartan to reduce the risk of cardiovascular events in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction. It is usually administered in conjunction with other heart failure therapies, in place of an ACE inhibitor or other ARB.
It is also used in combination with valsartan.
Background
Sacubitril is a prodrug neprilysin inhibitor used in combination with valsartan to reduce the risk of cardiovascular events in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction. It was approved by the FDA after being given the status of priority review for on July 7, 2015.
Sacubitril's active metabolite, LBQ657 inhibits neprilysin, a neutral endopeptidase that would typically cleave natiuretic peptides such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and c-type natriuretic peptide (CNP). ANP and BNP are released under atrial and ventricle stress, which activate downstream receptors leading to vasodilation, natriuresis and diuresis. Under normal conditions, neprilysin breaks down other vasodilating peptides and also vasoconstrictors such as angiotensin I and II, endothelin-1 and peptide amyloid beta-protein. Inhibition of neprilysin therefore leads to reduced breakdown and increased concentration of endogenous natriuretic peptides in addition to increased levels of vasoconstricting hormones such as angiotensin II.
Biological Activity
ahu-377 is an inhibitor of neprilysin with ic50 value of 5nm [1].ahu-377 and the angiotensin ii at1 receptor antagonist valsartan compose lcz696 in a 1:1 molar ratio. lcz696 is an angiotensin receptor neprilysin inhibitor. it can reduce blood pressure and may be a novel drug for the treatment of heart failure. ahu-377 is a prodrug, it can be converted by enzymatic cleavage of the ethyl ester into the active form lbq657. it is reported that ahu-377(30 and 100 mg/kg, po) can cause antihypertensive effect in a dose-dependent manner in dahl-ss rats. but in the doca-salt hypertensive rats, it shows a weak reduction [2, 3].
Pharmacokinetics
n a 7-day valsartan-controlled study in patients with reduced ejection fraction (HFrEF), administration of sacubitril + valsartan (Entresto) resulted in a significant non-sustained increase in natriuresis, increased urine cGMP, and decreased plasma MR-proANP and NT-proBNP compared to valsartan. In a 21-day study in HFrEF patients, it significantly increased urine ANP and cGMP and plasma cGMP, and decreased plasma NT-proBNP, aldosterone and endothelin-1. In clinical studies, this combination had no effect on QTc interval.
Metabolism
Sacubitril is metabolized to LBQ657 by esterases. A low concentration (<10%) of a hydroxyl metabolite has been identified in plasma.
References
[1] ksander gm, ghai rd, dejesus r, diefenbacher cg, yuan a, berry c, sakane y, trapani a. dicarboxylic acid dipeptide neutral endopeptidase inhibitors. j med chem. 1995 may 12;38(10):1689-700.
[2] voors aa, dorhout b, van der meer p. the potential role of valsartan + ahu377 ( lcz696 ) in the treatment of heart failure. expert opin investig drugs. 2013 aug;22(8):1041-7.
[3] laxminarayan g hegde, cecile yu, cheruvu madhavi et al. comparative efficacy of ahu-377, a potent neprilysin inhibitor, in two rat models of volume-dependent hypertension. bmc pharmacology 2011, 11(suppl 1):p33.
Properties of AHU-377
| Boiling point: | 656.9±55.0 °C(Predicted) |
| Density | 1.151±0.06 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | ≥41.1 mg/mL in DMSO; ≥21.45 mg/mL in H2O with ultrasonic; ≥26.85 mg/mL in EtOH with ultrasonic |
| form | oil |
| pka | 4.72±0.10(Predicted) |
| color | colorless |
Safety information for AHU-377
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for AHU-377
AHU-377 manufacturer
Sai Life Sciences Ltd
Optimus Pharma Pvt Ltd (Sekhmet Pharmaventures))
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
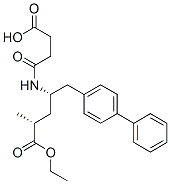
![(2R,4R)-5-(Biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid](https://img.chemicalbook.in/CAS/20180703/GIF/1012341-56-8.gif)
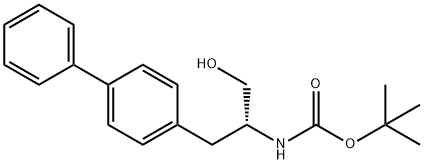
![(2R,4S)-ethyl 5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpentanoate](https://img.chemicalbook.in/CAS/20150408/GIF/149709-60-4.gif)
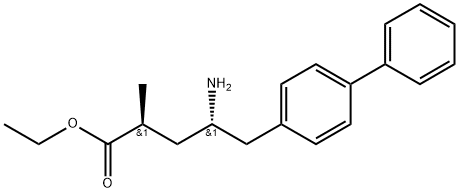
![(R,E)-5-([1,1'-biphenyl]-4-yl)-4-((tert-butoxycarbonyl)aMino)-2-Methylpent-2-enoic acid](https://img.chemicalbook.in/CAS/20150408/GIF/1012341-48-8.gif)
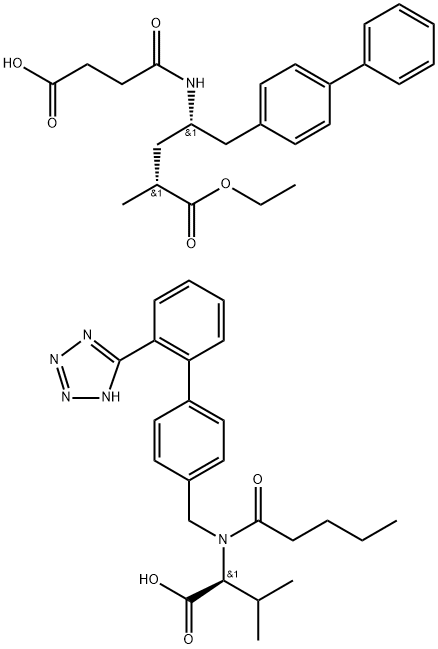
![(2S,4S)-5-(Biphenyl-4-yl)-4-[(tert-butoxycarbonyl)amino]-2-methylpentanoic acid](https://img.chemicalbook.in/CAS/20180703/GIF/1012341-52-4.gif)
![(2R,4S)-4-([1,1'-Biphenyl]-4-ylmethyl)-2-methyl-4-(2,5-dioxopyrrolidin-1-yl)butanoic acid](https://img.chemicalbook.in/CAS/20180702/GIF/1639970-62-9.gif)
You may like
-
 149709-62-6 Sacubitril 99%View Details
149709-62-6 Sacubitril 99%View Details
149709-62-6 -
 Sacubitril 98%View Details
Sacubitril 98%View Details
149709-62-6 -
 149709-62-6 98%View Details
149709-62-6 98%View Details
149709-62-6 -
 Sacubitril calcium salt, 98% CAS 149709-62-6View Details
Sacubitril calcium salt, 98% CAS 149709-62-6View Details
149709-62-6 -
 149709-62-6 Sacubitril 98%View Details
149709-62-6 Sacubitril 98%View Details
149709-62-6 -
 Sacubitril 149709-62-6 98%View Details
Sacubitril 149709-62-6 98%View Details
149709-62-6 -
 AHU-377 CAS 149709-62-6View Details
AHU-377 CAS 149709-62-6View Details
149709-62-6 -
 AHU-377 >95% CAS 149709-62-6View Details
AHU-377 >95% CAS 149709-62-6View Details
149709-62-6