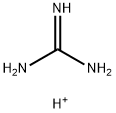
Agmatine sulfate
Synonym(s):N-(4-Aminobutyl)guanidine sulfate salt;1-Amino-4-guanidinobutane sulfate salt;4-Guanidinobutylamine sulfate salt
- CAS NO.:2482-00-0
- Empirical Formula: C5H16N4O4S
- Molecular Weight: 228.27
- MDL number: MFCD00013109
- EINECS: 219-617-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 12:07:08
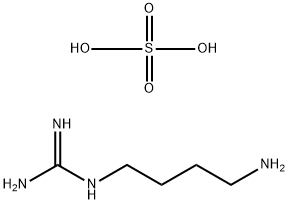
What is Agmatine sulfate ?
Description
Agmatine sulfate is the sulfate of the Agmatine. Agmatine is an aninoguanidine that is naturally derived from the arginine. It can exert modulatory effect on many molecular targets such as neurotransmitter systems, opioid analgesia, ion channels, and nitric oxide (NO) synthesis and polyamine metabolism. It has been also found that it can reverse the pain induced from inflammation, neuropathy, and spinal cord injury. Those effects make it a potential candidate for multiple pharmacological applications in the future.
Chemical properties
White to off-white powder
The Uses of Agmatine sulfate
Agmatine Sulfate is a a polyamine derived from L-arginine (A769500) decarboxylation and is a known putative endogenous neurotransmitter at imidazoline receptors. Agmatine sulfate displaces clonidine a t α2-adrenergic and at imidazoline receptors and was recently shown to exert some neuroprotective effects.
The Uses of Agmatine sulfate
Agmatine is an intermediate in the biosynthesis of the polyamines putrescine and spermine. Agmatine binds with high affinity to α2-adrenergic receptors and imidazoline binding sites and produces blockade of the NMDA-receptor associated cation channels. Agmatine is a competitive inhibitor of nitric oxide synthase activity owing to mimicry of the natural NOS substrate arginine.
What are the applications of Application
Agmatine sulfate is an L-arginine decarboxy analog with neurotransmiter activity
Biological Activity
Arginine metabolite that is synthesized within bovine brain and exhibits clonidine-displacing substance (CDS) activity. Putative endogenous ligand for the imidazoline binding site.
Biochem/physiol Actions
Putative endogenous neurotransmitter at imidazoline receptors; displaces clonidine at α2-adrenergic and at imidazoline receptors; blocks NMDA-activated ion channels in hippocampal neurons.
Purification Methods
Crystallise the salt from aqueous MeOH. The free base has m 101.5-103o, the gold chloride hydrochloride crystallises from H2O with m 223o(dec), and the picrate has m 236-238o. [Odo J Chem Soc Jpn 67 132 1946, Beilstein 4 I 420, 4 II 703, 4 III 575, 4 IV 1291.]
References
Fairbanks, C. A., et al. "Agmatine reverses pain induced by inflammation, neuropathy, and spinal cord injury." Proceedings of the National Academy of Sciences of the United States of America97.19(2000):10584.
Kolesnikov, Yuri, S. Jain, and G. W. Pasternak. "Modulation of opioid analgesia by agmatine." European Journal of Pharmacology296.1(1996):17-22.
Su, R. B., J. Li, and B. Y. Qin. "A biphasic opioid function modulator: agmatine. "Acta Pharmacologica Sinica24.7(2003):631.
Galea, Elena, et al. "Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine." Biochemical Journal 316 ( Pt 1).1(1996):247.
https://en.wikipedia.org/wiki/Agmatine
Properties of Agmatine sulfate
| Melting point: | 234-238 °C(lit.) |
| RTECS | ME8413000 |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | H2O: 50 mg/mL |
| form | powder |
| color | white to off-white |
| Water Solubility | Soluble in water, nearly insoluble in alcohol |
| Merck | 14,188 |
| BRN | 3918807 |
| InChI | InChI=1S/C5H14N4.H2O4S/c6-3-1-2-4-9-5(7)8;1-5(2,3)4/h1-4,6H2,(H4,7,8,9);(H2,1,2,3,4) |
| CAS DataBase Reference | 2482-00-0(CAS DataBase Reference) |
Safety information for Agmatine sulfate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Agmatine sulfate
| InChIKey | PTAYFGHRDOMJGC-UHFFFAOYSA-N |
| SMILES | C(CCCN)NC(N)=N.S(O)(O)(=O)=O |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Agmatine Sulfate CAS 2482-00-0View Details
Agmatine Sulfate CAS 2482-00-0View Details
2482-00-0 -
 Agmatine sulfate 98.00% CAS 2482-00-0View Details
Agmatine sulfate 98.00% CAS 2482-00-0View Details
2482-00-0 -
 Agmatine sulfate salt CAS 2482-00-0View Details
Agmatine sulfate salt CAS 2482-00-0View Details
2482-00-0 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
