Adefovir
Synonym(s):GS 0393;GS-393;P-[[2-(6-Amino-9H-purin-9-yl)ethoxy]methyl]phosphonic acid, 9-(2-phosphonylmethoxyethyl)adenine;PMEA
- CAS NO.:106941-25-7
- Empirical Formula: C8H12N5O4P
- Molecular Weight: 273.19
- MDL number: MFCD00866943
- EINECS: 600-789-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 16:48:35
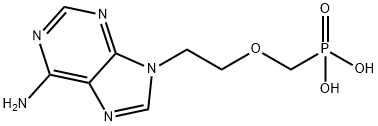
What is Adefovir?
Chemical properties
Pale Brown Solid
The Uses of Adefovir
Adefovir has been used to study its anti-retro viral effect on porcine endogenous retrovirus (PERV) activity.
The Uses of Adefovir
Used as an antiviral
What are the applications of Application
Adefovir is an antiviral compound
Definition
ChEBI: Adefovir is a member of the class of phosphonic acids that is methylphosphonic acid in which one of the methyl hydrogens has been replaced by a 2-(6-amino-9H-purin-9-yl)ethoxy group. An inhibitor of HIV-1 reverse transcriptase, the bis(t-butoxycarbonyloxymethyl) ester (dipivoxil ester) prodrug is used to treat chronic hepatitis B viral infection. It has a role as a HIV-1 reverse transcriptase inhibitor, a drug metabolite, an antiviral drug, a nephrotoxic agent and a DNA synthesis inhibitor. It is a member of 6-aminopurines, an ether and a member of phosphonic acids. It is functionally related to an adenine. It is a conjugate acid of an adefovir(1-).
Acquired resistance
It has a lower propensity to induce drug resistance than lamivudine. Clinical trials of patients receiving 48 weeks of therapy did not identify any cases of resistance. Longer courses yield resistant strains of HBV with mutations in the DNA polymerase gene; other rare variants of resistant strains have been identified. Lamivudine-resistant strains of HBV retain susceptibility to adefovir.
Pharmaceutical Applications
A nucleotide analog of adenosine monophosphate, administered orally as its prodrug, adefovir dipivoxil.
Biochem/physiol Actions
Adefovir is an antiviral drug that after intracellular conversion to adefovir diphosphate inhibits hepatitis B virus (HBV) DNA polymerase (reverse transcriptase).
Pharmacokinetics
Oral absorption: c. 60%
Cmax 10 mg/kg oral: 18.4 ng/mL
Plasma half-life: c. 7.5 h.
Volume of distribution: 392 mL/kg
Plasma protein binding: Not known
The prodrug is metabolized to adefovir, which is excreted by the kidneys and therefore requires dose adjustment in patients with impaired renal function. It does not induce cytochrome P450 at standard doses and does not influence the metabolism or plasma concentrations of the other licensed medications used in the treatment of hepatitis B.
Clinical Use
Treatment of chronic hepatitis B virus infection in patients >12 years of age
Side Effects
It is generally well tolerated, with headache, pharyngitis, abdominal pain and peripheral neuropathy being the most common side effects. Nephrotoxicity has been observed in some patients, with those receiving higher doses and longer courses of therapy at greater risk. Exacerbation of hepatitis has been reported in patients immediately following discontinuation of treatment. Most exacerbations occur within 12 weeks of stopping therapy, and elevations of alanine aminotransferase (ALT) up to 10 times the upper limit of normal can be observed in over 25% of patients. Lactic acidosis has been reported in a few patients and is an indication for immediate discontinuation.
Properties of Adefovir
| Melting point: | >260°C |
| Boiling point: | 632.5±65.0 °C(Predicted) |
| Density | 1.88 |
| storage temp. | -20°C |
| solubility | 0.1 M NaOH: ≥5mg/mL |
| form | powder |
| pka | pKa1 2.0, pKa2 6.8(at 25℃) |
| color | white to beige |
| CAS DataBase Reference | 106941-25-7(CAS DataBase Reference) |
Safety information for Adefovir
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
| Precautionary Statement Codes |
P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Adefovir
| InChIKey | SUPKOOSCJHTBAH-UHFFFAOYSA-N |
Adefovir manufacturer
KPS Chemicals And Pharmaceuticals
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
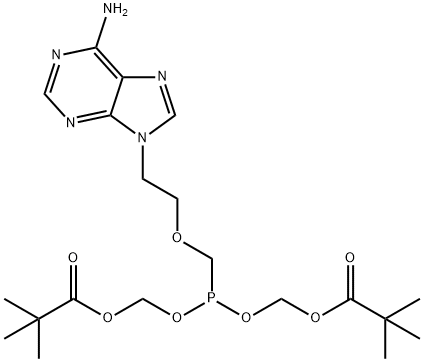
![Propanoic acid, 2,2-dimethyl-, [[[[2-(6-amino-9H-purin-9-yl)ethoxy]methyl]ethoxyphosphinyl]oxy]methyl ester](https://img.chemicalbook.in/CAS/GIF/142341-04-6.gif)




You may like
-
 106941-25-7 98%View Details
106941-25-7 98%View Details
106941-25-7 -
 Adefovir 97% CAS 106941-25-7View Details
Adefovir 97% CAS 106941-25-7View Details
106941-25-7 -
 Adefovir CAS 106941-25-7View Details
Adefovir CAS 106941-25-7View Details
106941-25-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1