ACTINONIN
Synonym(s):3-[[1-[(2-(Hydroxymethyl)-1-pyrrolidinyl)carbonyl]-2-methylpropyl]carbamoyl]octanohydroxamic acid
- CAS NO.:13434-13-4
- Empirical Formula: C19H35N3O5
- Molecular Weight: 385.5
- MDL number: MFCD00058569
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-30 15:45:59
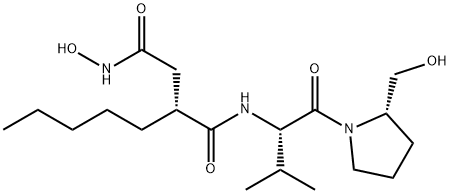
What is ACTINONIN?
Description
Actinonin (13434-13-4) is a potent hydroxamic acid inhibitor of aminopeptidases which displays analgesic effects by inhibiting enkephalin-degrading enzymes.1?Actinonin also inhibits peptide deformylase.2?Induces apoptosis and displays antitumor activity in vivo.3?Inhibits neutrophil collagenase and other MMPs.4 Induces mitochondrial proteotoxicity.5
Chemical properties
Off-White Solid
The Uses of ACTINONIN
(-)-Actinonin is a bioactive peptide and antibiotic that is a potent inhibitor of aminopeptidase M and leucine aminopeptidase. Actinonin has observed to be an inhibitor of peptide deformylase, meprin A, and thermolysin. Actinonin also inhibits tumor cell invasion and matrix degradation along with displayed antitumor activity in vitro and in vivo as well as acting as an apoptotic inducer.
The Uses of ACTINONIN
A potent in vivo inhibitor of collagenase
The Uses of ACTINONIN
Actinonin has been used as a control to inhibit meprin-β activity in C57BL/6 mice. It has also been used to study the high-affinity interaction of EcPDF with actinonin by 15N NMR spectroscopy and isothermal titration.
What are the applications of Application
Actinonin is an antibiotic shown to inhibit CD13, MMP-1,2,3,7,8,9,10,12, and MMP-13
General Description
Chemical structure: peptide
Biochem/physiol Actions
Actinonin has inhibitory action against peptide deformylase (PDF). It is effective against Gram-positive and fastidious Gram-negative microorganisms.
in vitro
actinonin showed an inhibitory effect in cell growth. the in vitro ic50 values of actinonin against nb4, hl6o human cell lines, akr mouse leukemia cells cd13-negative cell lines ra.ji and daudi human lymphoma were about 2-5 μg/ml. cell cycle analysis indicated that actinonin induced a g1 arrest in nb4 and hl6o cells. intracellular flow cytometry showed that actinonin could induce cell apoptosis in 20-35% of the cells [2]. actinonin dose-dependently inhibited the three forms (zn-, ni-, and fe-) of peptide deformylases from both s. aureus and e. coli bacteria. the ic50 values of actinonin were 90, 3, 0.8, and 11 nm for zn-pdf (e. coli), ni-pdf (e. coli), fe-pdf (e. coli), and ni-pdf (s. aureus), respectively [2]. actinonin is a tight binding inhibitor of e. coli ni-pdf with a ki of 0.3 nm [3].
in vivo
actinonin showed dose-dependent antitumor effects on akr leukemia, resulting in a survival advantage. in the syngeneic akr mouse leukemia model, treatment with 100 p.g actinonin daily for 3 days beginning at day 3 after transplantation showed significant antitumor effects [2].
storage
+4°C
References
1) Hachisu et al. (1987), Analgesic effect of actinonin, a new potent inhibitor of multiple encephalin degrading enzymes; Life Sci., 41 235 2) Lee et al. (2004), Human mitochondrial peptide deformylase, a new anticancer target of actinonin-based antibiotics; J. Clin. Invest., 114 1107 3) Xu et al. (1998), Antitumor activity of actinonin in vitro and in vivo; Clin. Cancer Res., 4 171 4) Sina et al. (2009), Cell-based evidence for aminopeptidase N/CD13 inhibitor actinonin targeting of MT1-MMP-mediated pro-MMP-2 activation; Cancer Lett., 279 171 5) Burman et al. (2017) Mitochondrial fission facilitate the selective mitophagy of protein aggregates; J. Cell Biol., 216 3231
Properties of ACTINONIN
| Melting point: | 137-139°C |
| Density | 1.139±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | Soluble in DMSO (up to 25 mg/ml) or in Ethanol (up to 20 mg/ml). |
| form | White to off-white powder. |
| pka | 9.19±0.20(Predicted) |
| color | White |
| BRN | 1555250 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 2 months. |
| CAS DataBase Reference | 13434-13-4(CAS DataBase Reference) |
Safety information for ACTINONIN
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ACTINONIN
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Actinonin CAS 13434-13-4View Details
Actinonin CAS 13434-13-4View Details
13434-13-4 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1