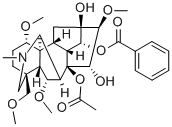
Aconitine
Synonym(s):Acetylbenzoylaconine
- CAS NO.:302-27-2
- Empirical Formula: C34H47NO11
- Molecular Weight: 645.74
- MDL number: MFCD00082375
- EINECS: 206-121-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
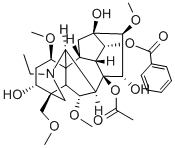
What is Aconitine?
Description
Aconitum plants (Wu Tou, Ranunculaceae family) have been widely used to treat various diseases, such as rheumatism, knee pain, herpes zoster, scabies, and other disorders in China for thousands of years. It was first described in Shen Nong’s Herbal Classic (the earliest classical work of Chinese herbs). Aconitine is the main active component in Aconitum plants.
Chemical properties
Off-White Solid
Physical properties
Appearance: solid. Solubility: barely soluble in water and soluble in organic solvents such as chloroform or diethyl ether. Its solubility in water and ethanol are 0.3?mg/mL and 35?mg/mL, respectively. Melting point: 203–204?°C (397–399?°F; 476–477?K). Optical rotation:D+17.3°
History
Preparations of Aconitum roots are employed in Chinese medicine for analgesic, antirheumatic, and neurological indications. In the Ming Dynasty, Chinese people had extracted the aconitine from the Aconitum plants. Aconitine is synthesized by the Aconitum plants via the terpenoid biosynthesis pathway (MEP chloroplast pathway)
The Uses of Aconitine
Neurotoxin. Activates tetrodotoxin-sensitive Na+ channels, inducing presynaptic depolarization, thus blocking the nerve action potential which, in turn, blocks the release of neurotransmitters and dec reases the end plate potential at the neuromuscular junction. Aconitine also blocks norepinephrine reuptake. In the heart, aconitine induces ventricular tachycardia after intracoronary injection. In c ultured ventricular myocytes, aconitine increases the duration of the action potential and induces the appearance of early after depolarization. Used in producing heart arrhythmia in experimental anim als.
The Uses of Aconitine
anesthetic (gastric), antipyretic, and cardiotoxin
The Uses of Aconitine
Aconitine occurs to the extent of 0.4–0.8%in dried tuberous roots of aconite or monkshood (Aconitum napellus L. and Ranunculaceae) found in India, North America,and Europe. It is used to produce heartarrhythmia in experimental animals and asan antipyretic agent.
What are the applications of Application
Aconitine is a lipid-soluble and potent neurotoxin that activates voltage gated, tetrodotoxin-sensitive sodium channels.
Definition
ChEBI: A diterpenoid that is 20-ethyl-3alpha,13,15alpha-trihydroxy-1alpha,6alpha,16beta-trimethoxy-4-(methoxymethyl)aconitane-8,14alpha-diol having acetate and b nzoate groups at the 8- and 14-positions respectively.
Indications
Aconitine was previously used as an antipyretic and analgesic. However, the clinical application of aconitine is limited by its high toxicity. Its lethal dose 50% (LD50) for mice is 1.8?mg/kg (orally) and 0.308?mg/kg (intraperitoneally).
Health Hazard
Aconitine is among the most toxic alkaloidsknown. Toxic doses are close to therapeuticdoses, and in humans as little as 2 mgmay cause death (Ferry and Vigneau 1983).An oral lethal dose of 28 mg/kg has alsobeen recorded (NIOSH 1986). The toxicsymptoms at low doses may be excitement,drowsiness, and hypermotility. The first signof poisoning from ingestion is a tingling,burning feeling on the lips, mouth, gums,and throat (Hodgson et al. 1988). This isfollowed by nervous disorders, anesthesia,loss of coordination, vertigo, hypersalivation,nausea, vomiting, and diarrhea. Toxic actionsof aconitine are very rapid. The crystallineform of this compound is much more toxicthan the amorphous aconitine. At a lethaldose, death may result from cardiorespiratoryfailure. Procaine may be an effective antidoteagainst aconitine poisoning.
Pharmacology
Aconitum was found to have neurotoxin. It could activate tetrodotoxin-sensitive Na+
channels, inducing presynaptic depolarization and blocking the nerve action potential and, in turn, blocking the release of neurotransmitters and decreasing the end
plate potential at the neuromuscular junction. Aconitine was also found to block
norepinephrine reuptake
In the heart, aconitine could induce ventricular tachycardia. In cultured ventricular myocytes, aconitine could induce the appearance of early after depolarization by
increasing the duration of the action potential.
Research with mouse nerve-hemidiaphragm muscle preparation found that aconitine at low concentrations (<0.1?μM) could increase the electrically evoked acetylcholine release causing an induced muscle tension. Action potentials were
generated more often at this concentration. While at higher concentrations
(0.3–3?μM), aconitine could decrease the electrically evoked acetylcholine release,
resulting in a decrease in muscle tension. Under the circumstance with high
concentration (0.3–3?μM) of aconitine, the sodium-ion channels were constantly
activated, and transmission of action potentials was suppressed, leading to the formation of non-excitable target cells or paralysis.
Clinical Use
In clinic, it can be used for treating and alleviating the symptoms caused by arthritis,
lumbocrural pain, and herpes zoster; however, it is utilized infrequently in clinical
practice due to its obvious side effects
According to a review of different reports of aconite poisoning in human beings,
the following clinical features such as neurological, cardiovascular, and gastrointestinal features were observed. The first symptom of aconitine poisoning appeared
approximately 20?min–2?h after oral intake, and they were paresthesia, sweating,
and nausea, which led to severe vomiting, colicky diarrhea, intense pain, and then
paralysis of the skeletal muscles. Following the onset of life-threatening arrhythmia,
including ventricular tachycardia and ventricular fibrillation, death finally occurred
as a result of respiratory paralysis or cardiac arrest.
Purification Methods
Crystallise it from EtOH, CHCl3 or toluene. [Beilstein 21/6 V 310.]
Properties of Aconitine
| Melting point: | 200-205 °C (dec.) |
| Boiling point: | 675.12°C (rough estimate) |
| alpha | D +17.3° (chloroform) |
| Density | 1.2181 (rough estimate) |
| refractive index | 1.6630 (estimate) |
| storage temp. | Refrigerator |
| solubility | Soluble in ethanol; |
| form | White to off-white powder. |
| pka | 5.88(at 25℃) |
| color | White |
| Water Solubility | Soluble in ethanol. Sparingly soluble in water |
| Merck | 13,120 |
| CAS DataBase Reference | 302-27-2 |
| NIST Chemistry Reference | Aconitine(302-27-2) |
Safety information for Aconitine
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
Computed Descriptors for Aconitine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Aconitine CAS 302-27-2View Details
Aconitine CAS 302-27-2View Details
302-27-2 -
 Aconitine CAS 302-27-2View Details
Aconitine CAS 302-27-2View Details
302-27-2 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
