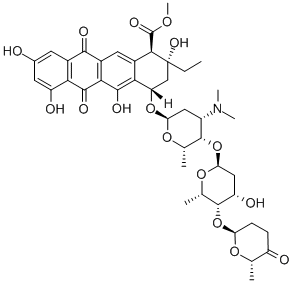
Aclarubicin
- CAS NO.:57576-44-0
- Empirical Formula: C42H53NO15
- Molecular Weight: 811.88
- MDL number: MFCD00866250
- EINECS: 260-824-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-25 23:13:37
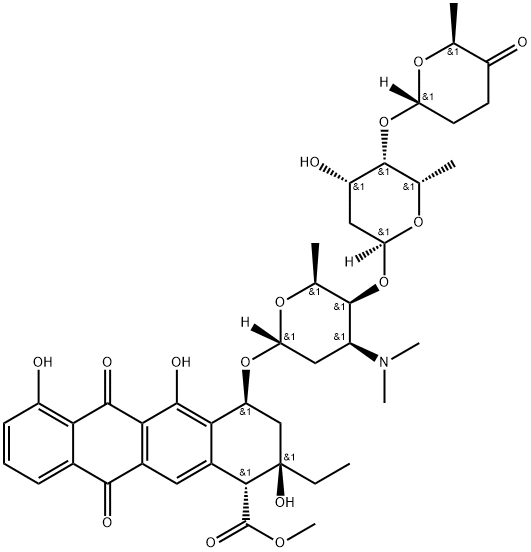
What is Aclarubicin?
Description
Aclarubicin was found in the culture broth of Streptomyces galilaeus MA144-M1 by Umezawa et al. of the Institute of Microbial Chemistry in 1975. It was produced along with structurally related compounds showing antileukemic activity and named aclacinomycin A. Sanraku-Ocean cooperated in isolating aclacinomycin A as a yellow crystalline powder and evaluated its strong antileukemic activity and low cardiac toxicity. Its generic name was changed to aclarubicin on the recommendation of the World Health Organization.
Originator
Aclacinon ,Yamanouchi ,Japan ,1981
The Uses of Aclarubicin
Aclarubicin is an anthracycline antibiotic. It is used in the treatment of cancer.
The Uses of Aclarubicin
Antineoplastic.
What are the applications of Application
Aclacinomycin A is an anticancer anthracycline drug and non-peptidic inhibitor of CTRL and calpain.
Definition
ChEBI: An anthracycline antibiotic that is produced by Streptomyces galilaeus and also has potent antineoplastic activity.
Manufacturing Process
100 ml of this medium was sterilized at 120°C for 15 min in a 500 ml
Sakaguchi-shaking flask which was inoculated from an agar slant culture of
Streptomyces galilaeus MA144-M1 by platinum loop. Incubation proceeded for
48 hr at 28°C on a reciprocal shaker. 10 L of the previously sterilized medium
in a 20 L stainless steel jar fermenter were aseptically inoculated with 200 ml
of the above seed cultures. Fermentation was carried out at 28°C for 32 hours
with agitation (240 rpm) and aeration (5 L/min). The cultured broth obtained
was adjusted to pH 4.5, mixed with an adsorbent siliceous earth material and
filtered from the mycelium. The filtrate and cake obtained thereby were
extracted separately. The cake was suspended in acetone (3 L/kg wet cake),
stirred for 2 hr and filtered, and the cake was further extracted with acetone
once again. The extracts thus obtained were evaporated to one-tenth volume
in vacuum. The culture filtrate was adjusted to pH 6.8 and extracted twice
with one-third volume of ethyl acetate, and the ethyl acetate extracts were
concentrated to one-tenth volume in vacuum.
Twenty grams of the resulting oily substances were mixed with 20 grams of
silicic acid (Mallinckrodt Chemical Co.), applied to a column 40 cm in length
and 4.5 cm in diameter filled with silicic acid, and eluted with a benzeneacetone-
methanol mixture. The initial eluate which eluted with a 1:1:0
mixture was discarded and the active fractions eluted with 1:3:0 and 1:3:0.3
mixtures were collected and concentrated to dryness in vacuum. 11.5 g of this
crude substance was then dissolved in a small amount of ethyl acetate and
applied to the same silicic acid column as above. After discarding the initial
eluates by the 1:1 and 2:1 benzene-acetone mixtures, aclacinomycin B
fractions were first eluted with the above mixtures of 1:3 and 1:5 ratio, and
aclacinomycin A fractions were then eluted with the 1:5:0.5 and 1:5:1 benzene-acetone-methanol mixtures. The eluates were dried over anhydrous
sodium sulfate and concentrated to dryness in vacuum. 4.8 g of crude
aclacinomycin A and 3.5 g of aclacinomycin B were obtained as yellow powder.
2.0 g of crude aclacinomycin A obtained as above were dissolved in a small
amount of chloroform, applied to a column 20 cm in length and 20 cm in
diameter filled with 30 g of silicic acid. After eluting off the pigments
containing aglycone and aclacinomycin B and other impurities with chloroform
and 1.5% methanol-containing chloroform, aclacinomycin A fractions were
eluted with 2% methanol-containing chloroform, and concentrated to dryness
in vacuum. 53 mg of yellow powder of aclacinomycin A was obtained. Its
melting point was 129°C to 135°C.
Therapeutic Function
Antitumor, Antibiotic
Biological Activity
aclacinomycin a is a dual inhibitor of topoisomerase i and ii [1]. aclacinomycin a is an anticancer drug which can reduce the tumor with minimal damage to normal cells. aclacinomycin a shows potency against a wide variety of solid tumours and haematological malignancies. in a549, hepg2 and mcf-7 cells, aclacinomycin a shows cytotoxic activity with ic50 values of 0.27μm, 0.32μm and 0.62μm, respectively. aclacinomycin a induces cell apoptosis in these cells and the effects change to be necrosis when the incubation time is prolonged. aclacinomycin a is demonstrated to increase the activity of both caspase-3 and caspase-8, thus inducing the activation of parp. apart from that, as an inhibitor of opoisomerases, aclacinomycin a is found to induce dna damage in v79 and irs-2 cells. aclacinomycin a is used to treat acute leukaemias, lymphomas and other solid tumors through its inhibition of topo ii [1, 2].
Safety Profile
Poison by ingestion,intraperitoneal, subcutaneous, and intravenous routes. Anexperimental teratogen. Other experimental reproductiveeffects. Mutation data reported. An eye and subcutaneousirritant. When heated to decomposition it emits toxicfumes of
Enzyme inhibitor
This non-peptidic aclacinomycin antibiotic (FW = 811.88 g/mol; CAS CAS 57576-44-0; Source: strain of Streptomyces galilaeus), also known as aclarubicin, induces DNA strand scission. Target(s): nitric oxide synthase; RNA biosynthesis; DNA polymerase I; RNA polymerase, Escherichia coli; reverse transcriptase, avian myeloblastosis virus; Na+/K+-exchanging ATPase; Ca2+-transporting ATPase; cyclicnucleotide phosphodiesterase; electron transport and oxidative phosphorylation, mitochondrial; DNA helicase; DNA topoisomerase II; 20S proteasome, chymotrypsin-like activity; DNA topoisomerase I; 3'-5' DNA helicase, Plasmodium falciparum.
References
[1] hajji n, mateos s, pastor n, domínguez i, cortés f. induction of genotoxic and cytotoxic damage by aclarubicin, a dual topoisomerase inhibitor. mutat res. 2005 may 2;583(1):26-35.
[2] rogalska a, szwed m, jó wiak z. aclarubicin-induced apoptosis and necrosis in cells derived from human solid tumours. mutat res. 2010 jul 19;700(1-2):1-10.
Properties of Aclarubicin
| Melting point: | 151-153° (dec) |
| Boiling point: | 756.05°C (rough estimate) |
| alpha | D24 -11.5° (c = 1 in methylene chloride) |
| Density | 1.2261 (rough estimate) |
| refractive index | 1.6220 (estimate) |
| storage temp. | 2-8°C |
| solubility | Dichloromethane (Slightly), DMSO (Slightly), Methanol (Slightly) |
| pka | 6.41±0.70(Predicted) |
| form | Yellow powder with orange cast. |
| color | Yellow powder |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 57576-44-0(CAS DataBase Reference) |
Safety information for Aclarubicin
Computed Descriptors for Aclarubicin
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1