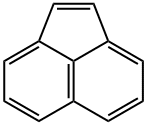
Acenaphthene
Synonym(s):1,8-Ethylenenaphthalene;Acenaphthene
- CAS NO.:83-32-9
- Empirical Formula: C12H10
- Molecular Weight: 154.21
- MDL number: MFCD00003807
- EINECS: 201-469-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
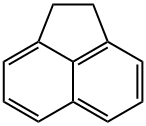
What is Acenaphthene?
Description
Acenaphthene is a tricyclic aromatic hydrocarbon and crystalline solid at ambient temperature. Acenaphthene does not dissolve in water but is soluble in many organic solvents. Acenaphthene is a component of crude oil and a product of combustion. Acenaphthene occurs in coal tar produced during the high-temperature carbonisation or coking of coal. It is used as a dye intermediate in the manufacture of some plastics and as an insecticide and fungicide. Acenaphthene is a component of crude oil and a product of combustion which may be produced and released to the environment during natural fires. Emissions from petroleum refining, coal tar distillation, coal combustion, and diesel-fuelled engines are major contributors of acenaphthene to the environment. Acenaphthene is an environmental pollutant and has been detected in cigarette smoke, automobile exhausts, and urban air; in effluents from petrochemical, pesticide, and wood preservative industries; and in soils, groundwater, and surface waters at hazardous waste sites. This compound is one among a number of polycyclic aromatic hydrocarbons (PAHs) on U.S. EPA’s (Environmental Protection Agency) priority pollutant list.
Chemical properties
white or pale yellow crystalline powder
Chemical properties
Acenaphthene is a tricyclic aromatic hydrocarbon, crystalline solid at ambient tempera- ture. Acenaphthene does not dissolve in water, but is soluble in many organic solvents. Acenaphthene occurs in coal tar produced during high temperature carbonization or cok- ing of coal. It is used as a dye intermediate in the manufacture of some plastics and as an insecticide and fungicide. Acenaphthene is a component of crude oil and a product of combustion that may be produced and released into the environment during natural fi res. Emissions from petroleum refi ning, coal tar distillation, coal combustion, and diesel- fueled engines are major contributors of acenaphthene to the environment. Acenaphthene is an environmental pollutant and has been detected in cigarette smoke, automobile exhausts, and urban air; in effl uents from petrochemical, pesticide, and wood preservative industries; and in soils, groundwater, and surface waters at hazardous waste sites. This compound is one of a number of polycyclic aromatic hydrocarbons on the US EPA’s prior- ity pollutant list.
Chemical properties
Acenaphthene is a white combustible, crystalline solid. PAHs are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons
Physical properties
White crystalline solid or orthorhombic bipyramidal needles from alcohol. Coal tar-like odor. The lowest odor threshold concentration in water that may result in rejection of contaminated water ranged from 0.02 to 0.22 ppm (Lillard and Powers, 1975). In Wisconsin, the taste and odor threshold concentration in water that is nontoxic to humans is 20 μg/L (ATSDR, 1995).
The Uses of Acenaphthene
Acenaphthene occurs in petroleum bottoms and is used as a dye intermediate, insecticide, and fungicide and in manufacturing plastics.
The Uses of Acenaphthene
Polycyclic aromatic hydrocarbons as carcinogenic
The Uses of Acenaphthene
Dye intermediate; manufacture of plastics; insecticide; fungicide.
Definition
ChEBI: A polycyclic aromatic hydrocarbon derived from naphthalene by the addition of an ethylene bridge connecting C-1 and C-8.
Definition
A colorless crystalline derivative of naphthalene, used in producing some dyes.
Definition
acenaphthene: A colourless crystallinearomatic compound, C12H10;m.p. 95°C; b.p. 278°C. It is an intermediatein the production of somedyes.
Synthesis Reference(s)
Synthetic Communications, 14, p. 1119, 1984 DOI: 10.1080/00397918408059644
General Description
White needles. Melting point 93.6°C. Soluble in hot alcohol. Denser than water and insoluble in water. Hence sinks in water. May irritate skin and mucous membranes. Emits acrid smoke and irritating fumes when heated to decomposition. Derived from coal tar and used to make dyes, pharmaceuticals, insecticides, fungicides, and plastics.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Acenaphthene is incompatible with strong oxidizing agents. Incompatible with ozone and chlorinating agents. Forms crystalline complexes with desoxycholic acid .
Health Hazard
Exposures to acenaphthene cause poisoning and include symptoms such as irritation to the skin, eyes, mucous membranes, and upper respiratory tract. Studies on labora- tory animals orally exposed to acenaphthene showed loss of body weight, peripheral blood changes (unspecifi ed), increased aminotransferase levels in blood serum, and mild morphological damage to the liver and kidneys. In chronic exposures, acenaph- thene is known to cause damage to the kidneys and liver. Acenaphthene is irritating to the skin and mucous membranes of humans and animals. Oral exposure of rats to ace- naphthene for 32 days produced peripheral blood changes, mild liver and kidney dam- age, and pulmonary effects. However, detailed studies with acenaphthene in humans are limited.
Health Hazard
Carcinogenicity of acenaphthene in animalsis not established. Tests for mutagenicity havegiven inconclusive results.
Fire Hazard
Flash point data for Acenaphthene are not available. Acenaphthene is probably combustible.
Safety Profile
Moderately toxic by intraperitonealroute. Mutation data reported.Incompatible with strongoxidizing agents, ozone, chlorinating agents. When heatedto decomposition it emits acrid smoke and irritating vapors.
Potential Exposure
Acenaphthene occurs naturally in coal tar and in coal tar produced during the high-temperature carbonization or coking of coal; coal tar distilling; petroleum processing; shale oil processing. It is used as an intermediate for dyes, fungicides, insecticides, herbicides, pharmaceuticals, plant growth hormones; 1,8 naphthalic acid; in the manufacture of some plastics; and has been detected in cigarette smoke and gasoline exhaust condensates; a constituent of coal tar creosote, asphalt, and diesel fuel. It has been used as an polyploidy agent.
Source
Detected in groundwater beneath a former coal gasification plant in Seattle, WA at a
concentration of 180 g/L (ASTR, 1995). Acenaphthene is present in tobacco smoke, asphalt,
combustion of aromatic fuels containing pyridine (quoted, Verschueren, 1983). Acenaphthene was
detected in asphalt fumes at an average concentration of 18.65 ng/m3 (Wang et al., 2001). Present
in diesel fuel and corresponding aqueous phase (distilled water) at concentrations of 100 to 600
mg/L and 4 to 14 g/L, respectively (Lee et al., 1992).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
625. Average acenaphthene concentrations reported in water-soluble fractions of unleaded gasoline, kerosene, and diesel fuel were 1, 2, and 6 g/L, respectively.
Acenaphthene occurs naturally in coal tar. Based on laboratory analysis of 7 coal tar samples,
acenaphthene concentrations ranged from 350 to 12,000 ppm (EPRI, 1990). Detected in 1-yr aged
coal tar film and bulk coal tar at concentrations of 5,800 and 5,900 mg/kg, respectively (Nelson et
al., 1996). A high-temperature coal tar contained acenaphthene at an average concentration of 1.05
wt % (McNeil, 1983). Lee et al. (1992a) equilibrated 8 coal tars with distilled water at 25 °C. The
maximum concentration of acenaphthene observed in the aqueous phase was 0.3 mg/L.
Nine commercially available creosote samples contained acenaphthene at concentrations
ranging from 9,500 to 110,000 mg/kg (Kohler et al., 2000).
Acenaphthene was detected in a diesel-powered medium duty truck exhaust at an emission rate
of 19.3 μg/km (Schauer et al., 1999) and is a component in cigarette smoke. Acenaphthene was
detected in soot generated from underventilated combustion of natural gas doped with 3 mole %
toluene (Tolocka and Miller, 1995).
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without
catalytic converters were 6.55 and 177 μg/km, respectively (Schauer et al., 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic
compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rates of acenaphthene were 2.02 mg/kg of pine burned, 1.15 mg/kg of oak burned, and 0.893
mg/kg of eucalyptus burned.
Under atmospheric conditions, a low rank coal (0.5–1 mm particle size) from Spain was burned
in a fluidized bed reactor at seven different temperatures (50 °C increments) beginning at 650 °C.
The combustion experiment was also conducted at different amounts of excess oxygen (5 to 40%)
and different flow rates (700 to 1,100 L/h). At 20% excess oxygen and a flow rate of 860 L/h, the
amount of acenaphthene emitted ranged from 1,272.4 ng/kg at 650 °C to 6,800.0 ng/kg at 750 °C.
The greatest amount of PAHs emitted was observed at 750 °C (Mastral et al., 1999).
Typical concentration of acenaphthene in a heavy pyrolysis oil is 1.6 wt % (Chevron Phillips,
May 2003).
Environmental Fate
Biological. When acenaphthene was statically incubated in the dark at 25 °C with yeast extract
and settled domestic wastewater inoculum, significant biodegradation with rapid adaptation was
observed. At concentrations of 5 and 10 mg/L, 95 and 100% biodegradation, respectively, were
observed after 7 d (Tabak et al., 1981). A Beijerinckia sp. and a mutant strain (Beijerinckia sp.
strain B8/36) cooxidized acenaphthene to the following metabolites: 1,2-acenaphthenediol,
acenaphthene-quinone, and a compound tentatively identified as 1,2-dihydroxyacenaphthylene
(Schocken and Gibson, 1984). The fungus Cunninghamella elegans ATCC 36112 degraded
approximately 64% acenaphthene added within 72 h of incubation. Metabolites identified and
their respective yields were 6-hydroxyacenaphthenone (24.8%), 1,2-acenaphthenedione (19.9%),
trans-1,2-dihydroxyacenaphthene (10.3%), 1,5-dihydroxyacenaphthene (2.7%), 1-acenaphthenol
(2.4%), 1-acenaphthenone (2.1%), and cis-1,2-dihydroxyacenaphthene (1.8%) (Pothuluri et al.,
1992). A recombinant strain of Pseudomonas aeruginosa PAO1(pRE695) degraded acenaphthene
via mono-oxygenation to 1-acenaphthenol which was converted to 1-acenaphthenone and cis- and
trans-1,2-dihydroxyacenaphthenes. The two latter compounds were subsequently converted to 1,2-
acenaphthoquinone which oxidized to naphthalene-1,8-dicarboxylic acid (Selifonov et al., 1996).
In a soil-water system, acenaphthene did not biodegrade under anaerobic conditions. Under
denitrification conditions, acenaphthene (water concentration 400 μg/L) degraded to nondetectable
levels in 40 d. In both studies, the acclimation period was approximately 2 d (Mihelcic
and Luthy, 1988).
Photolytic. Fukuda et al. (1988) studied the photodegradation of acenaphthene and alkylated
naphthalenes in distilled water and artificial seawater using a high-pressure mercury lamp. Based
upon a rate constant of 0.23/h, the photolytic half-life of acenaphthene in water is 3 h. Behymer
and Hites (1985) determined the effect of different substrates on the rate of photooxidation of
acenaphthene using a rotary photoreactor equipped with a 450-W medium pressure mercury lamp
(λ = 300–410 nm). The photolytic half-lives of acenaphthene absorbed onto silica gel, alumina,
and fly ash were 2.0, 2.2, and 44 h, respectively. The estimated photooxidation half-life of
acenaphthene in the atmosphere via OH radicals is 0.879 to 8.79 h (Atkinson, 1987).
Chemical/Physical. Ozonation in water at 60 °C produced 7-formyl-1-indanone, 1-indanone, 7-
hydroxy-1-indanone, 1-indanone-7-carboxylic acid, indane-1,7-dicarboxylic acid, and indane-1-
formyl-7-carboxylic acid (Chen et al., 1979). Wet oxidation of acenaphthene at 320 °C yielded
formic and acetic acids (Randall and Knopp, 1980). The measured rate constant for the gas-phase
reaction of acenaphthene with OH radicals is 8.0 x 10-11 cm3/molecule·sec (Reisen and Arey,
2002).
Shipping
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
It has also been purified by chromatography from CCl4 on alumina with *benzene as eluent [McLaughlin & Zainal J Chem Soc 2485 1960]. [Beilstein 5 IV 1834.]
Incompatibilities
Ozone and strong oxidizing agents, including perchlorates, chlorine, fluorine, and bromine
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Incineration or permanganate oxidation
Properties of Acenaphthene
| Melting point: | 90-94 °C(lit.) |
| Boiling point: | 279 °C(lit.) |
| Density | 1.06 |
| vapor density | 5.32 (vs air) |
| vapor pressure | 10 mm Hg ( 131 °C) |
| refractive index | 1.6048 |
| Flash point: | 135 °C |
| storage temp. | Store below +30°C. |
| solubility | chloroform: 50 mg/mL, clear |
| form | crystalline |
| color | brown-beige |
| Water Solubility | 0.000347 g/100 mL |
| Merck | 14,28 |
| BRN | 386081 |
| Henry's Law Constant | 3.47, 6.21, 10.8, 18.3, and 28.2 at 4.1, 11.0, 18.0, 25.0, and 31.0 °C, respectively (Bamford et al.,
1998) |
| Dielectric constant | 3.0 |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 83-32-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Acenaphthene(83-32-9) |
| IARC | 3 (Vol. 92) 2010 |
| EPA Substance Registry System | Acenaphthene (83-32-9) |
Safety information for Acenaphthene
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P501:Dispose of contents/container to..… |
Computed Descriptors for Acenaphthene
Acenaphthene manufacturer
ARRAKIS INDUSTRIES LLP
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Acenaphthene CAS 83-32-9View Details
Acenaphthene CAS 83-32-9View Details
83-32-9 -
 Acenaphthene CAS 83-32-9View Details
Acenaphthene CAS 83-32-9View Details
83-32-9 -
 Acenaphthene, pract CAS 83-32-9View Details
Acenaphthene, pract CAS 83-32-9View Details
83-32-9 -
 Acenaphthene CAS 83-32-9View Details
Acenaphthene CAS 83-32-9View Details
83-32-9 -
 Acenaphthene, for HPLC 98%+ CAS 83-32-9View Details
Acenaphthene, for HPLC 98%+ CAS 83-32-9View Details
83-32-9 -
 Acenaphthene CAS 83-32-9View Details
Acenaphthene CAS 83-32-9View Details
83-32-9 -
 Acenaphthene 96% CAS 83-32-9View Details
Acenaphthene 96% CAS 83-32-9View Details
83-32-9 -
 ACENAPHTHENE For Synthesis CAS 83-32-9View Details
ACENAPHTHENE For Synthesis CAS 83-32-9View Details
83-32-9