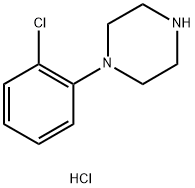
Acaprazine
- CAS NO.:55485-20-6
- Empirical Formula: C15H21Cl2N3O
- Molecular Weight: 330.257
- MDL number: MFCD00868769
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-23 13:36:13
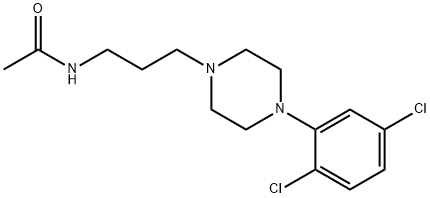
What is Acaprazine?
Originator
Acaprazine,ZYF Pharm Chemical
Manufacturing Process
2 methods of producing of 1-(3-acetylaminopropyl)-4-(2,5-dichlorophenyl)
piperazine:
1. A mixture formed by N-(3-chloropropyl)phthalimide (23.0 g), 1-(2,5-
dichlorophenyl)piperazine (24.0 g), anhydrous sodium carbonate (14.0 g) and
toluene (130 ml) is boiled under stirring for 30 h. The solution is then cooled,
filtered, concentrated to dryness, and the residue is treated with water and
ether. The ethereal extract is washed, dried and concentrated to dryness. The residue is treated with ethyl acetate and transformed into hydrochloride with
ethanolic HCl. N-[3-[4-(2,5-Dichlorophenyl)-1-piperazinyl]propyl]phthalimide
hydrochloride (30.0 g) is obtained, melting point 233°C (dec.).
N-[3-[4-(2,5-Dichlorophenyl)-1-piperazinyl]propyl]phthalimide (15.0 g) and
99% hydrazinehydrate (2 ml) are heated, under reflux, for 90 min. 5 N HCl
(115 ml) is then added and the solution is heated for 30 min under reflux.
The solution is cooled, filtered and concentrated to small volume. The residue
is diluted with water, made alkaline by means of a solution of NaOH and
extracted with CH2Cl2. The organic layer is dried, evaporated and distilled
under reduced pressure. 7.0 g of 1-(3-aminopropyl)-4-(2,5-dichlorophenyl)
piperazine are obtained.
1-(3-Aminopropyl)-4-(2,5-dichlorophenyl)piperazine (50.0 g) is dissolved in
CH2Cl2, and the solution is heated under reflux. Acetic anhydride (25 ml)
dissolved in CH2Cl2, (500 ml) is introduced under stirring in about 1 h. The
solution is heated for 1 h and is evaporated to dryness in a rotating
evaporator. The is treated with H2O, thus obtaining a solid which is separated,
washed and dried at 50°C. This solid is then dissolved in ethyl acetate (about
350 ml), treated with charcoal and crystallized. 1-(3-Acetylaminopropyl)-4-
(2,5-dichlorophenyl)piperazine is obtained in an almost quantitative yield,
melting point 114°C.
2. Chlorosuccinimide (5.4 g) is added to a solution of 4-(m-chlorophenyl)-1-
piperazinopropionitrile (5.0 g) in CH2Cl2 (100 ml). The solution is heated for a
few hours and, after removal of the solvent, it is chromatographed on an
alumina column. The solution is eluted with a 1:1 mixture of cyclohexane and
benzene containing 0.3 % of triethylamine. 4-(2,5-Dichlorophenyl)-1-
piperazinopropionitrile (2.0 g) is thus obtained.
4-(2,5-Dichlorophenyl)-1-piperazinopropionitrile (20.0 g), acetic anhydride
(100 ml), anhydrous sodium sulfate (12.0 g) and a 50% solution of Ni-Raney
(6 ml) are hydrogenated at 60.0 pounds per inch at 60°C in a Parr apparatus.
After about half an absorption of hydrogen is complete. The reaction mixture
is cooled, the is filtered and the solution is concentrated to small volume in a
rotating evaporator. The residue is treated with NaOH solution and extracted
several times CH2Cl2. The organic layer is washed, dried and evaporated. The
residue is crystallized from ethyl acetate. 14.0 g of the 1-(3-
acetylaminopropyl)-4-(2,5-dichlorophenyl)piperazine are obtained, melting
point 114°C.
Therapeutic Function
Adrenergic blocker, Tranquilizer
Safety information for Acaprazine
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4