ablukast
- CAS NO.:96566-25-5
- Empirical Formula: C28H34O8
- Molecular Weight: 498.57
- MDL number: MFCD00864781
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
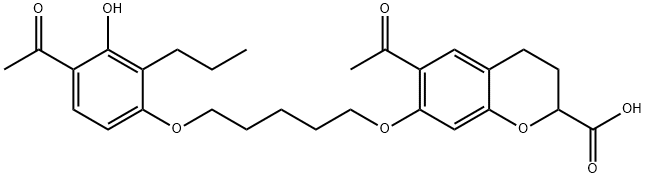
What is ablukast?
Originator
Ulpax,Roche
The Uses of ablukast
Anti-asthmatic (leukotriene antagonist).
Manufacturing Process
A solution of 109.8 g (0.75 mol) of diethyl oxalate and 65 g (0.427 mol) of
2',4'-dihydroxyacetophenone in 100 mL of EtOH was added slowly under Ar,
with cooling, to a stirred solution of NaOEt (from 40 g of Na and 550 mL of
EtOH). The mixture was stirred at 50°C for 3 h, cooled to room temperature,
and poured into a separatory funnel containing 500 mL of 2 N HCl. It was
extracted with CH2CH2 washed with 500 mL of saturated NaHCO3, dried and
evaporated to give a red oil, which was dissolved in 250 mL of EtOH and 10
mL of conc. HCl. The mixture was boiled under reflux for 1 h, cooled to ca.
10°C and the product was collected by filtration. It was washed with some
EtOH followed by hexane to give 86.0 g (86% yield) of ethyl 7-hydroxy-4-oxo-
4H-1-benzopyran-2-carboxylate: m.p. 218-223°C. Crystallization of a portion
from hot AcOH gave an analytical sample: m.p. 221-223°C.
A solution of 80 g (0.34 mol) of ethyl 7-hydroxy-4-oxo-4H-1-benzopyran-2-
carboxylate in 60 mL of AcOH and 275 mL of THF was hydrogenated over 4.0
g of 10% Pd on charcoal at 45°C and 65 psi. After hydrogen absorption
ceased, the catalyst was removed by filtration and the solvents were
evaporated under reduced pressure. Crystallization from CCl4 gave 65 g
(85%) of ethyl (R,S)-3,4-dihydro-7-hydroxy-2H-1-benzopyran-2-carboxylate:
m.p. 80-82°C.
A stirred mixture of 65 g (0.293 mol) of ethyl (R,S)-3,4-dihydro-7-hydroxy-
2H-1-benzopyran-2-carboxylate in 650 mL of AcOH, 1.5 mL of acetic
anhydride, and 65 mL of BF3·OEt2 was heated at reflux for 18 h and
evaporated. To the residue was added 700 mL of water, and the mixture was
stirred at room temperature for 1.0 h. The product was collected by filtration and washed with hexane. It was then dissolved in 900 mL of MeOH, treated
with 6.6 g of p-toluenesulfonic acid, boiled under reflux for 18 h, and cooled
to 0°C. The product was collected by filtration to give 52 g (70% yield) of
ethyl (R,S)-3,4-dihydro-7-hydroxy-2H-1-benzopyran-2-carboxylate: m.p. 140-
142°C.
A 1 L, 3-necked, round-bottomed flask equipped with a mechanical stirrer and
an Ar bubbler was charged with 37.5 g (0.179 moles) of 5-bromo-1-pentanyl
acetate, 350 mL of anhyd. DMSO, 40.7 g (0.163 mol) of ethyl (R,S)-3,4-
dihydro-7-hydroxy-2H-1-benzopyran-2-carboxylate, and 51.0 g (0.369 mol) of
powdered potassium carbonate. The mixture was stirred at room temperature
for 18 h, poured into 1.0 L of water and extracted into EtOAc (2*1 L). The
extract was washed with 1 L of brine, dried and evaporated. The residue was
dissolved in 200 mL of ether, cooled to 57°C and, with stirring, diluted with
petroleum ether. The product was collected by filtration, washed with a little
1:1 ether-petroleum ether (b.p. 40-60°C) and dried to give 60.0 g (97%) of
methyl (R,S)-6-acetyl-3,4-dihydro-7-((5-acetoxypentyl)oxy-2H-1-benzopyran-
2-carboxylate: m.p.51-53°C.
A solution of 72.08 g (0.19 mol) of 5-bromo-1-pentanyl acetate in 1.4 L of
MeOH was treated with 38 mL of a 1.0 molar solution of tetrabutylammonium
hydroxide and the mixture was stirred at room temperature for 3.0 h, 3.0 mL
of AcOH was added and the solution was evaporated at 35°C. The residue was
dissolved in 400 mL of EtOAc and the solution was washed with saturated
NaHCO3, brine, dried, and evaporated to give 60.45 g (94% yield) of the
intermediate hydroxy ester (an analytical sample may be obtained by
crystallization from 70% EtOAc in hexane, m.p. 58-61°C. A stirred solution of
60.25 g of the hydroxyester in 700 mL of EtOAc was cooled to 5°C and
treated with 75.5 mL (3 equiv.) of triethylamine and 32.6 mL (2.35 equiv.) of
methanesulfonyl chloride. The mixture was stirred at 6°C for 2.0 h,
transferred to a separatory funnel and washed sequentially with water, 2 N
HCl, and brine. Concentration of the EtOAc to ca. 300 mL and dilution cooled
to 0°C and treated with 75.5 mL (3 equiv.) of triethylamine and 32.6 mL
(2.35 equiv.) of methanesulfonyl chloride. The mixture was stirred at 6°C for
2.0 h transferred to a separatory funnel and washed sequentially with water, 2
N HCl, and brine. Concentration of the EtOAc to ca. 300 mL and dilution with
250 mL of hexane led to crystallization (0°C, 18 h). The product was collected
by filtration and washed with some cold hexane - EtOAc (1:1) to give 66 g
(84% yield) of methyl (R,S)-6-acetyl-3,4-dihydro-7-[5-
[(methylsufonyl)oxy]pentyloxy]-2H-1-benzopyran-2-carboxylate: m.p. 73-
76°C.
A mixture of 66.13 g (0.159 mol) of methyl (R,S)-6-acetyl-3,4-dihydro-7-[5-
[(methylsufonyl)oxy]pentyloxy]-2H-1-benzopyran-2-carboxylate, 30.99 g
(0.159 mol) of 1-[2,4-dihydroxy-3-propylphenyl)ethanone, 33.07 g (0.239
mol) of pulverized potassium carbonate, 5.16 g (15.9 mmol) of tris(3,6-
dioxahepyl)amine in 900 mL of toluene was stirred under Ar at reflux for 6 h
and then at room temperature overnight. The mixture was poured into 300
mL of water and the organic phase was separated, washed with brine, dried
and evaporated to give 84.3 g of methyl (R,S)-6-acetyl-7-[5-(4-acetyl-3-
hydroxy-2-propylphenoxy)pentoxy]-3,4-dihydro-2H-1-benzopyran-2-
carboxilate. Crystallization from MeOH (0°C, 18 h) gave 66 g (81% yield),
m.p. 77-80°C.
A stirred solution of 55.82 g (0.109 mol) of methyl (R,S)-6-acetyl-7-[5-(4-
acetyl-3-hydroxy-2-propylphenoxy)pentoxy]-3,4-dihydro-2H-1-benzopyran-2-
carboxylate in 725 mL of MeOH was treated with 4.45 g (0.111 mol) of NaOH
in 20 mL of water and the mixture was stirred at reflux for 1.25 h. It was
cooled, concentrated to a volume of ca 360 mL, diluted with 310 mL of ether
and left at 0°C overnight. The product was collected by filtration, dried in to
give 45.76 g of (R,S)-6-acetyl-7-[[5-(4-acetyl-3-hydroxy-2-
propylphenoxy)pentyl]oxy]-3,4-dihydro-2H-H-benzopyran-2-carboxylic acid
sodium salt (Ablukast) as the monohydrate. A further 12.27 g of product was
obtained from the mother liquor to give a total yield of 99%.
Therapeutic Function
Antiallergic, Anti-asthmatic
Properties of ablukast
| Melting point: | 113-115 °C |
| Boiling point: | 730.8±60.0 °C(Predicted) |
| Density | 1.219±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO : 100 mg/mL (200.58 mM; Need ultrasonic) |
| pka | 2.92±0.20(Predicted) |
| form | Solid |
| color | White to off-white |
Safety information for ablukast
Computed Descriptors for ablukast
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1