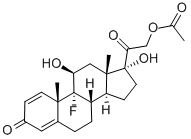
9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 21-[tricyclo[3.3.1.13,7]dec-1-ylformate]
- CAS NO.:40242-27-1
- Empirical Formula: C33H43FO6
- Molecular Weight: 554.695
- EINECS: 254-855-1
- SAFETY DATA SHEET (SDS)
![9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 21-[tricyclo[3.3.1.13,7]dec-1-ylformate] Structural](https://img.chemicalbook.in/CAS/GIF/40242-27-1.gif)
What is 9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 21-[tricyclo[3.3.1.13,7]dec-1-ylformate]?
Originator
Betamethasone,Zhejiang Xianju Pharmaceutical Co., Ltd.
Manufacturing Process
3 Methods of producing of betamethasone 21-adamantane-1'-carboxylate:
1. A suspension of betamethasone (740.0 mg) in dioxan (20 ml) was treated
with adamantane carboxylic acid (1.96 g) and trifluorcacetic anhydride (0.75
ml). The mixture was stirred at room temperature for 23 h during which time
the steroid completely dissolved. Addition of sodium bicarbonate (2.0 g) and
water gave a waxy semi-solid which was separated from the supernatant
liquid by decantation. Water and a little methanol were added to the solid and
the resulting granular material was removed by filtration and washed well with
water. Fractional crystallization from methanol afforded adamantane carboxylic
anhydride as the less soluble component and betamethasone 21-adamantane-
1'-carboxylate as the more soluble component.
2. 9α-Fluoro-11β,17-dihydroxy-21-iodo-16β-methylpregna-1,4-diene-3,20-
dione (76.65 g) was dissolved in warm acetone (400 ml) and then
adamantanecarboxylic acid (54.0 g) and triethylamine (52.5 ml) was added
and washed in with more acetone (100 ml). The solution was refluxed for 1 h
and then poured with good stirring into cold water (2.5 L). Filtration of the
precipitated material and recrystallisation from aqueous methanol with
charcoaling afforded betamethasone 21-admantane-1'-carboxylate showing
extensive melting 245°-250°C.
3. A solution of betamethasone (1.0 g) in dry tetrahydrofuran (40 ml) was
treated with adamantane carbonyl chloride (about 2.2 equivalents) in dry
tetrahydrofuran (5 ml) and then pyridine (0.8 ml) was added. The mixture
was refluxed for 6 h and then most of the solvent was boiled off and the
residue extracted with chloroform to afford a froth. The ether soluble portion
of this froth was dissolved in chloroform and extracted repeatedly with dilute
sodium bicarbonate solution. Evaporation of the chloroform layer gave a froth
which was further purified by chromatography and crystallisation from
chloroform-petroleum ether to yield betamethasone 21-adamantane-1'-
carboxylate melting point 256°-259°C (dec.).
Therapeutic Function
Glucocorticoid
Safety information for 9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 21-[tricyclo[3.3.1.13,7]dec-1-ylformate]
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 4-Hydrazinobenzoic acid Electrolytic Iron Powder Fmoc-Val-Cit-PAB 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 4-HYDROXY BENZYL ALCOHOL 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) S-2-CHLORO PROPIONIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 1-(4-Methylphenylsulfonyl)-1H-1,2,3-benzotriazole 1-(2-Chlorobenzyl)-4-nitro-1H-pyrazole 1-(2-Nitrophenyl)-4-phenylpiperazineRelated products of tetrahydrofuran
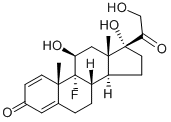
![9-fluoro-11beta,17-dihydroxy-16beta-methylpregna-1,4-diene-3,20-dione 21-[tricyclo[3.3.1.13,7]dec-1-ylformate]](https://img.chemicalbook.in/CAS/GIF/40242-27-1.gif)

You may like
-
 55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 2 2-BIS(2-HYDROXYETHOXY)-1 1-BINAPHTHYL 99%View Details
55441-95-7 -
 181228-33-1 99%View Details
181228-33-1 99%View Details
181228-33-1 -
 Ste-Glu-AEEA-AEEA-OSUView Details
Ste-Glu-AEEA-AEEA-OSUView Details
1169630-40-3 -
 1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 Fmoc-His-Aib-OH TFA 98%View Details
1446013-08-6 -
 127464-43-1 99%View Details
127464-43-1 99%View Details
127464-43-1 -
 Chloro Uracil 99%View Details
Chloro Uracil 99%View Details
1820-81-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 13162-05-5 99%View Details
13162-05-5 99%View Details
13162-05-5