9-Fluorenone
Synonym(s):9-Fluorenone
- CAS NO.:486-25-9
- Empirical Formula: C13H8O
- Molecular Weight: 180.2
- MDL number: MFCD00001141
- EINECS: 207-630-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 14:19:35
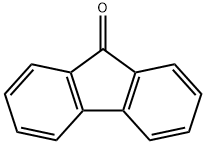
What is 9-Fluorenone?
Description
9-fluorenone is an important intermediate for organic synthesis. It can be used to manufacture a variety of fine chemicals, mainly used as a modifier for the production of polymer materials, bisphenol fluorene, fluorenyl benzoxazine resin, acrylic resin, polyester, polycarbonate and epoxy resin. In the laboratory, fluorene was used as the raw material, dimethyl sulfoxide as the solvent, sodium hydroxide as the catalyst and oxygen as the oxidant. The oxidizing reaction was carried out by a tower packing reactor. The reaction solution was cooled and filtered to obtain a crude fluorenone. The content of crude fluorenone is 93%. We can recover 94% of the solvent and part of the crude fluorenone through distillation of the filtrate. The crude fluorenone is purified by directional crystallization to obtain yellow flaky fluorenone that have a purity of 99.8% or more.
Chemical properties
yellow flakes, chips or crystalline powder; Soluble in ethanol and ether, insoluble in water.
The Uses of 9-Fluorenone
9-Fluorenone is used in the preparation of antimalarial drugs. It is a fluorene derivative. Further, it is used in functional polymer and in dyes.
What are the applications of Application
9-Fluorenone is 9-Fluorenone is a polycyclic aromatic ketone that has photosensitizing properties and also has the ability to undergo electrochemical polymerization. 9-Fluorenone is used in industry as an initiator in visible-light cured resin systems. 9-Fluorenone has potentially toxic effects on humans, as exposure can cause DNA mutations.
Definition
ChEBI: Fluoren-9-one is the simplest member of the class fluoren-9-ones that is 9H-fluorene bearing an oxo substituent at position 9. It has a role as a fungal xenobiotic metabolite.
What are the applications of Application
9-Fluorenone has been extensively used as a precursor to synthesize a variety of organic electronic materials. Some of the general examples are:
Synthesis of host for the blue and green phosphorescent organic light emitting diodes (PHOLEDs).
Synthesis of fluorene-based molecular motors.
Synthesis of open-shell Chichibabin′s hydrocarbons as potential organic spintronic materials.
It also acts as a sensitizer in the formation of picene via photosensitization of 1,2-di(1-naphthyl)ethane.
Preparation
Fluorenone can be produced by catalytic oxidation of fluorene, or of fluorene fractions in the presence of a quarternary ammonium salt, or by catalytic oxidative cracking (oxicracking) of a suitable aromatic.
Synthesis Reference(s)
The Journal of Organic Chemistry, 60, p. 3934, 1995 DOI: 10.1021/jo00118a002
Synthetic Communications, 6, p. 285, 1976 DOI: 10.1080/00397917608063524
Purification Methods
Crystallise 9-fluorenone from absolute EtOH, MeOH or *benzene/pentane. [Ikezawa J Am Chem Soc 108 1589 1986.] Also recrystallise it twice from toluene and sublime it in a vacuum [Saltiel J Am Chem Soc 108 2674 1986]. It can be distilled under high vacuum. [Beilstein 7 H 465, 7 III 2330, 7 IV 1629.]
Properties of 9-Fluorenone
| Melting point: | 80-83 °C (lit.) |
| Boiling point: | 342 °C (lit.) |
| Density | 0,9 g/cm3 |
| vapor pressure | 0.005-0.2Pa at 20-50℃ |
| refractive index | 1.6309 (estimate) |
| Flash point: | 163 °C |
| storage temp. | Store below +30°C. |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Crystalline Powder or Flakes |
| color | Yellow |
| Water Solubility | insoluble |
| BRN | 1636531 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 486-25-9(CAS DataBase Reference) |
| NIST Chemistry Reference | 9H-Fluoren-9-one(486-25-9) |
| EPA Substance Registry System | 9-Fluorenone (486-25-9) |
Safety information for 9-Fluorenone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 9-Fluorenone
| InChIKey | YLQWCDOCJODRMT-UHFFFAOYSA-N |
9-Fluorenone manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
![9,9-Bis[4-(2-acryloyloxyethyloxy)phenyl]fluorene](https://img.chemicalbook.in/CAS/GIF/161182-73-6.gif)

You may like
-
 486-25-9 9-Fluorenone, 98% 99%View Details
486-25-9 9-Fluorenone, 98% 99%View Details
486-25-9 -
 9-Fluorenone CAS 486-25-9View Details
9-Fluorenone CAS 486-25-9View Details
486-25-9 -
 9-Fluorenone CAS 486-25-9View Details
9-Fluorenone CAS 486-25-9View Details
486-25-9 -
 9-Fluorenone CAS 486-25-9View Details
9-Fluorenone CAS 486-25-9View Details
486-25-9 -
 9-Fluorenone CAS 486-25-9View Details
9-Fluorenone CAS 486-25-9View Details
486-25-9 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
