9-Fluorenol
Synonym(s):9-Fluorenol
- CAS NO.:1689-64-1
- Empirical Formula: C13H10O
- Molecular Weight: 182.22
- MDL number: MFCD00001135
- EINECS: 216-879-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
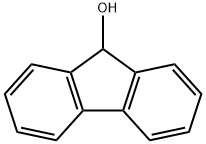
What is 9-Fluorenol?
Chemical properties
white powder
The Uses of 9-Fluorenol
9-Fluorenol is used in Organic Synthesis, Pharmaceuticals, Agrochemicals and Dyestuffs.
Definition
ChEBI: 9-Fluorenol is a member of the class of hydroxyfluorenes that is 9H-fluorene substituted by a hydroxy group at position 9 (the non-aromatic carbon). It has a role as an animal metabolite. It is a member of hydroxyfluorenes and a secondary alcohol.
What are the applications of Application
9-Fluorenol is a wake-promoting agent and is considered a next-generation anti-drowsiness drug. It is also a potential environmental carcinogen.
9-Fluorenol is a dopamine reuptake inhibitor with IC50 of 9 μM, and a major metabolite of a compound developed as a wakefulness-promoting agent.
Preparation
Synthesis of 9-Fluorenol: A 5-g sample of 9-fluorenone is dissolved in 30 mL of warm ethanol in a 100-mL beaker. To this solution is added, dropwise, 10 mL of a reducing reagent, freshly prepared, which consists of 200 mg sodium methoxide,10 mL methanol, and 0.4 g sodium borohydride.This solution is allowed to stand undisturbed for 10-20 min. A color change is observed as the reaction proceeds from the yellow 9-fluorenone to the white 9-fluorenol.The product is precipitated by the addition of 50 mL of water and neutralized with 0.1 M HCl, vacuum filtered, and washed with cold water to remove any residual inorganic salt formed by the excess of the sodium borohydride reagent and hydrochloric acid.The dried crude product is sufficiently pure for characterization by melting point, infrared spectra,and NMR.The crude product melting point is 153°C, with a product yield of 95-100%.
A Synthesis of 9-Fluorenol: Sodium Borohydride Reduction of 9-Fluorenone
Synthesis Reference(s)
The Journal of Organic Chemistry, 57, p. 6313, 1992 DOI: 10.1021/jo00049a045
Tetrahedron Letters, 13, p. 343, 1972
Synthesis
9-Fluorenol is prepared by reacting 9-fluorenone with THF solution of phosphazene under the action of boron catalyst.Add
9-fluorenone (0.8 mmol), a 0.2 M THF solution of phosphazene (80 μL,
0.016 mmol) and a 0.0762 M THF solution of boron catalyst (420 μL, 0.032
mmol) to a scintillation vial with magnetic stir bar in a glove box.
Place the scintillation vial in a Parr reactor. Seal the reactor.
Pressurize the reactor with hydrogen gas. Heat reaction mixture at 75°C
(inside the reactor). Stir the reaction mixture (1000 rpm) for 20 hours
at 75°C. Cool the mixture to room temperature and vent the Parr reactor
to obtain 9-hydroxyfluoren. Analyze the reaction mixture by 1H NMR spectroscopy using CDCl3 as solvent.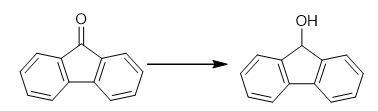 Fig the synthetic method of 9-Fluorenol
Fig the synthetic method of 9-Fluorenol
Properties of 9-Fluorenol
| Melting point: | 153-154 °C(lit.) |
| Boiling point: | 275.62°C (rough estimate) |
| Density | 1.0368 (rough estimate) |
| refractive index | 1.5994 (estimate) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Crystalline Powder |
| pka | 13.34±0.20(Predicted) |
| color | Cream |
| Water Solubility | Insoluble in water. |
| BRN | 1869799 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 1689-64-1(CAS DataBase Reference) |
| NIST Chemistry Reference | 9H-fluoren-9-ol(1689-64-1) |
| EPA Substance Registry System | 9-Hydroxyfluorene (1689-64-1) |
Safety information for 9-Fluorenol
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment |
Computed Descriptors for 9-Fluorenol
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 9-Fluorenol CAS 1689-64-1View Details
9-Fluorenol CAS 1689-64-1View Details
1689-64-1 -
 9-Fluorenol CAS 1689-64-1View Details
9-Fluorenol CAS 1689-64-1View Details
1689-64-1 -
 9-Hydroxyfluorene CAS 1689-64-1View Details
9-Hydroxyfluorene CAS 1689-64-1View Details
1689-64-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8