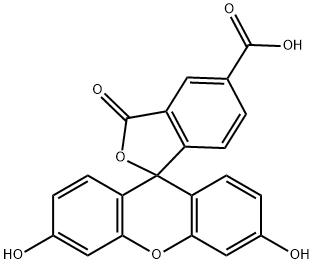
6-Carboxyfluorescein
Synonym(s):6-Carboxyfluorescein;6-FAM;FLUOS
- CAS NO.:3301-79-9
- Empirical Formula: C21H12O7
- Molecular Weight: 376.32
- MDL number: MFCD00036873
- EINECS: 636-503-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
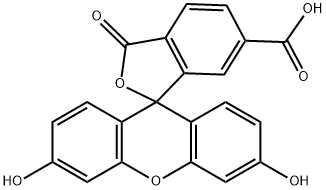
What is 6-Carboxyfluorescein?
Chemical properties
Orange Solid
The Uses of 6-Carboxyfluorescein
6-Carboxyfluorescein is derivative of fluorescein giving stable derivative upon conjugation with biopolymers.It has been used to check the plasma membrane integrity in sperm cells.
What are the applications of Application
6-Carboxyfluorescein is a carboxylated fluorescein dye
Preparation
1,2,4-Benzenetricarboxylic anhydride (also called 4- carboxyphthalic anhydride, 25.0 g, 0.13 mol) was added to a solution of 1,3-dihydroxybenzene (also called resorcinol, 28.6 g, 0.26 mol) in methane sulfonic acid (1M). An air condenser was attached to the flask and the reaction was heated at 85 oC in an open vessel for 24 h. After cooling to room temperature, the reaction mixture was poured into 7 volumes of ice/water. An orange-yellow precipitate formed; this was collected by filtration and dried in an oven at 200 oC. This residue was recrystallized two times from methanol/hexane to give 1.0 g of 6- carboxyfluorescein methanesulfonic acid adduct preferentially crystallizes from MeOH/hexanes. The mother liquors from this procedure were collected, the solvent was removed in vacuo, and the residues were recrystallized two times from ethanol/hexanes to give 3.2 g of 5-carboxyfluorescein methanesulfonic acid adduct preferentially crystallizes from EtOH/hexanes. Finally, the mother liquors from this experiment were combined, evaporated to dryness, and recrystallized two times from methanol/hexanes to give another 3.0 g of 6-carboxyfluorescein methanesulfonic acid adduct preferentially crystallizes from MeOH/hexanes, making a total yield of 4.0 g, 40 %. Careful dropwise addition of conc. HCl(aq) to solutions of these methanesulfonic esters preferentially crystallizes from EtOH/hexanes and preferentially crystallizes from MeOH/hexanes in 4M sodium hydroxide gave 5-carboxyfluorescein and 6-carboxyfluorescein, respectively in near quantitative yield.
Definition
ChEBI: 6-carboxyfluorescein is a monocarboxylic acid. It derives from a fluorescein (lactone form).
What are the applications of Application
6-Carboxyfluorescein is a useful reagent for the preparation of hydrolytically stable fluorescent conjugates and is a useful starting material for the synthesis of other fluorescein-derives reagent. Fluorescein is the most common fluorescent derivatization reagent for labeling biomolecules. In addition to its relatively high absorptivity, excellent fluorescence quantum yield, and good water solubility, fluorescein has an excitation maximum that closely matches the 488 nm spectral line of the argon-ion laser.
Biochem/physiol Actions
6-Carboxyfluorescein diacetate is a membrane permeable compound. It can be hydrolysed by intracellular esterases to form a membrane impermeable bright green fluorescent molecule, 6-carboxyfluorescein.
Properties of 6-Carboxyfluorescein
| Melting point: | >300 °C(lit.) |
| Boiling point: | 736.4±60.0 °C(Predicted) |
| Density | 1.73±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO: soluble |
| form | Solid |
| pka | 3.3, 4.6, 6.4, 7.0(at 25℃) |
| color | Yellow to orange |
| PH Range | Weak green ' uorescence (6.0) to strong green ' uourescence (7.2) |
| λmax | 492nm |
| BRN | 54341 |
| Major Application | Diagnosis of hematologic cancer, nongastric diseases, detection of genetically modified wheat, chromosomes, gene expression, nucleic acid, hepatitis A virus, avian influenza virus subtype H5 and H5N1, SARS virus, herpex simplex virus |
| Biological Applications | Diagnosing bladder cancer; evaluating corneal endothelial barrier function; as a substrate for measuring protein kinase activity, methyltransferase activity, phospholipase activity, rhodanese activity,sulfotransferase activity;use in ophthalmology |
| CAS DataBase Reference | 3301-79-9(CAS DataBase Reference) |
| EPA Substance Registry System | Spiro[isobenzofuran-1(3H),9'-[9H]xanthene]-6-carboxylic acid, 3',6'-dihydroxy-3-oxo- (3301-79-9) |
Safety information for 6-Carboxyfluorescein
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 6-Carboxyfluorescein
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 6-Carboxyfluorescein CAS 3301-79-9View Details
6-Carboxyfluorescein CAS 3301-79-9View Details
3301-79-9 -
 6-Carboxyfluorescein CAS 3301-79-9View Details
6-Carboxyfluorescein CAS 3301-79-9View Details
3301-79-9 -
 6-Carboxyfluorescein CAS 3301-79-9View Details
6-Carboxyfluorescein CAS 3301-79-9View Details
3301-79-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1