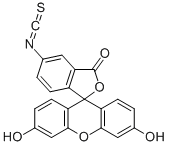
6-Aminofluorescein
Synonym(s):Fluoresceinamine isomer II
- CAS NO.:51649-83-3
- Empirical Formula: C20H13NO5
- Molecular Weight: 347.32
- MDL number: MFCD00005051
- EINECS: 257-334-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 16:23:17
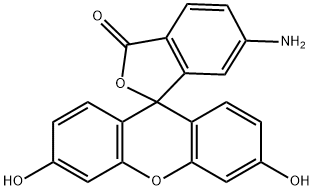
What is 6-Aminofluorescein?
Description
6-Aminofluorescein (6-AF) is a new fluorescence dye with bright colors and high fluorescence intensity. Its chemical structure history contains a nitrogen group and a fluorescent group. The presence of the fluorescent component enables 6-Aminofluorescein to emit a distinct fluorescent signal when excited, making it valuable for fluorescent labeling and imaging. It is widely used in biological science research, chemical analysis and fluorescence microscopy.
Chemical properties
Yellow Solid
The Uses of 6-Aminofluorescein
Glycosaminoglycuronans react with 6-aminofluorescein to yield fluorescent derivatives.
The Uses of 6-Aminofluorescein
Fluorescent labeling reagent for proteins. Glycosaminoglycuronans react with 6-aminofluorescein to yield fluorescent derivatives. Used in the fluorescent antibody technique for rapid identification of pathogens.
What are the applications of Application
6-Aminofluorescein is a fluorescent labeling reagent for proteins
Biological Functions
6-Aminofluorescein has been demonstrated to be useful fluorescent labeling reagent for fullerene-based liposome nanostructures termed "buckysomes". 6-aminofluorescein dissolved in 0.1 N NaOH can be quantified from a maximum absorbance at 490 nm. Decarboxylated 6-aminofluorescein dissolved 6 N HCl (prepare by constant boiling at 110° C for 24 h) can be quantified from a maximum absorbance at 454 nm. Aldehyde groups are formed by oxidateive damage to proteins, which can be detected by conjugation to decarboxylated 6-aminofluorescein. 6-Aminofluorescein has been used to determine its ability to potentiate the antiviral activity of poly r (A-U).
References
[1] BHARATE G Y, QIN H, FANG J. Poly(Styrene-Co-Maleic Acid)-Conjugated 6-Aminofluorescein and Rhodamine Micelle as Macromolecular Fluorescent Probes for Micro-Tumors Detection and Imaging[J]. Journal of Personalized Medicine, 2022, 12. DOI:10.3390/jpm12101650.
[2] Müller, Werner EG, and Heinz Christoph Schr?der, eds. Biological Response Modifiers—Interferons, Double-Stranded RNA and 2′, 5′-Oligoadenylates. Vol. 14. Springer Science & Business Media, 2012.
Properties of 6-Aminofluorescein
| Melting point: | 285 °C (dec.)(lit.) |
| Boiling point: | 481.89°C (rough estimate) |
| Density | 1.3724 (rough estimate) |
| refractive index | 1.5180 (estimate) |
| storage temp. | 2-8°C |
| solubility | methanol: soluble1mg/mL, clear, yellow to very dark yellow-orange |
| form | Solid |
| pka | 9.34±0.20(Predicted) |
| color | Yellow to Orange |
| BRN | 51708 |
| Stability: | Light and Moisture Sensitive |
| InChI | InChI=1S/C20H13NO5/c21-10-1-4-13-16(7-10)20(26-19(13)24)14-5-2-11(22)8-17(14)25-18-9-12(23)3-6-15(18)20/h1-9,22-23H,21H2 |
| CAS DataBase Reference | 51649-83-3(CAS DataBase Reference) |
Safety information for 6-Aminofluorescein
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 6-Aminofluorescein
| InChIKey | YOAWSYSKQHLFPM-UHFFFAOYSA-N |
| SMILES | C12(C3=C(C=C(O)C=C3)OC3=C1C=CC(O)=C3)C1=C(C=CC(N)=C1)C(=O)O2 |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 6-Aminofluorescein (isomer II) CAS 51649-83-3View Details
6-Aminofluorescein (isomer II) CAS 51649-83-3View Details
51649-83-3 -
 6-Aminofluorescein CAS 51649-83-3View Details
6-Aminofluorescein CAS 51649-83-3View Details
51649-83-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
