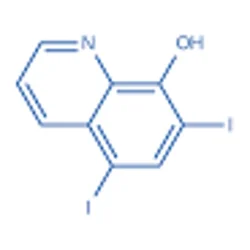
5,7-Diiodo-8-quinolinol
Synonym(s):5,7-Diiodo-8-hydroxyquinoline;5,7-Diiodo-8-quinolinol
- CAS NO.:83-73-8
- Empirical Formula: C9H5I2NO
- Molecular Weight: 396.95
- MDL number: MFCD00006789
- EINECS: 201-497-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-22 19:45:46
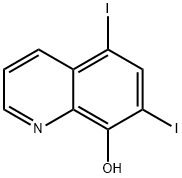
What is 5,7-Diiodo-8-quinolinol?
Originator
Diiodohydroxyquinoline,Adco Co.
The Uses of 5,7-Diiodo-8-quinolinol
Iodoquinol is an amebocide used against E. histolytica, and it is active against both cysts and trophozoites that are localized in the lumen of the intestine. It is considered the drug of choice for treating asymptomatic or moderate forms of amebiasis. The mechanism of action is unknown. Iodoquinol is used for diseases caused by moderate intestinal amebiasis. Synonyms of this drug are diquinol, iodoxin, diiodoquin, amebaquin, and others.
The Uses of 5,7-Diiodo-8-quinolinol
It acts as an amoebicidal and so used in the treatment of amoebiasis, balantidiasis (an infection caused by protozoa).
Background
Diiodohydroxyquinoline, also known as uidoquinol and iodoquinol, is a quinoline derivative that can be used in the treatment of amoebiasis. The exact mechanism of action is unknown. Iodoquinol is not currently available in any FDA-approved products.
Indications
Used in the treatment of amoebiasis.
Indications
Iodoquinol (diiodohydroxyquin, Yodoxin, Moebiquin)
is a halogenated 8-hydroxyquinoline derivative whose
precise mechanism of action is not known but is thought
to involve an inactivation of essential parasite enzymes.
Iodoquinol kills the trophozoite forms of E. histolytica,
B. coli, B. hominis, and Dientamoeba fragilis.
Iodoquinol is absorbed from the gastrointestinal
tract and is excreted in the urine as glucuronide and sulfate
conjugates. Most of an orally administered dose is
excreted in the feces. Iodoquinol has a plasma half-life
of about 12 hours.
Iodoquinol is the drug of choice in the treatment of
asymptomatic amebiasis and D. fragilis infections. It is
also used in combination with other drugs in the treatment
of other forms of amebiasis and as an alternative
to tetracycline in the treatment of balantidiasis.
Adverse reactions are related to the iodine content
of the drug; the toxicity is often expressed as skin reactions,
thyroid enlargement, and interference with thyroid
function studies. Headache and diarrhea also occur.
Chronic use of clioquinol, a closely related agent,
has been linked to a myelitislike illness and to optic atrophy
with permanent loss of vision.
Definition
ChEBI: Iodoquinol is a monohydroxyquinoline that is quinolin-8-ol in which the hydrogens at positions 5 and 7 are replaced by iodine. It is considered the drug of choice for treating asymptomatic or moderate forms of amoebiasis. It has a role as an antiamoebic agent, an antibacterial agent, an antiseptic drug and an antiprotozoal drug. It is a monohydroxyquinoline and an organoiodine compound.
Manufacturing Process
5,7-Diiodo-8-quinolinol widely used as an intestinal antiseptic, especially as an
antiamebic agent. It is also used topically in other infections and may cause
CNS and eye damage. It is known by very many similar trade names
worldwide.
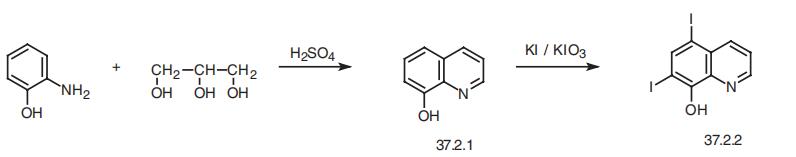
0.01 mol 8-oxychinoline and 0.01 mol salicylic acid were dissolved in 500 ml
of water and then 0.05 mol potassium iodide was added. The mixture was
heated to temperature 90°-100°C. After that 0.01 mol of KIO3 by little tiles
was added. The next tile was added after a disappearence of discharging
iodine. Then 10 ml 2 N HCl was added. The solid product was fallen, filtered
off, washed with hot water and in 0.25 N NaOH dissolved. The solution was
filtered and the clear filtrate precipitated with a very little excess of HCl. The
product 5,7-diiodo-8-quinolinol was filtered, washed with hot water and dried.
MP: 200°-250°C (with decomposition).
brand name
Quinadome (Bayer); Yodoxin (Glenwood).
Therapeutic Function
Antibacterial
Flammability and Explosibility
Non flammable
Clinical Use
5,7-Diiodo-8-quinolinol, 5,7-diiodo-8-hydroxyquinoline,or diiodohydroxyquin (Yodoxin, Diodoquin, Diquinol) is ayellowish to tan microcrystalline, light-sensitive substancethat is insoluble in water. It is recommended for acute andchronic intestinal amebiasis but is not effective in extraintestinaldisease. Because a relatively high incidence of topicneuropathy has occurred with its use, iodoquinol should notbe used routinely for traveler’s diarrhea.
Safety Profile
Poison by ingestion and intravenous routes. Human systemic effects by ingestion: eye effects. Mutation data reported. When heated to decomposition it emits very toxic fumes of Iand Nox
Synthesis
Iodoquinol, 5,7-diiodo-8-quinolinol (37.2.2), is made by iodination of 8-oxyquinoline (37.2.1) using a mixture of potassium iodide/potassium iodate. The initial 8-hydroxyquinolin (37.2.1) is made from 2-aminophenol and glycerol in the presence of sulfuric acid and nitrobenzene (Skraup synthesis).
Metabolism
Not Available
Purification Methods
It crystallises from xylene and is dried at 70o in a vacuum. [Beilstein 21 II 58.]
Properties of 5,7-Diiodo-8-quinolinol
| Melting point: | >200 °C (dec.) (lit.) |
| Boiling point: | 370.95°C (rough estimate) |
| Density | 2.3013 (estimate) |
| storage temp. | Refrigerator |
| solubility | DMSO (Slightly, Heated), Ethyl Acetate (Slightly, Heated) |
| form | Solid |
| pka | pKa 8.0 (Uncertain) |
| color | Pale Yellow to Light Beige |
| Water Solubility | 1g/L at 26℃ |
| Merck | 14,5042 |
| CAS DataBase Reference | 83-73-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 5,7-Diiodo-8-hydroxyquinoline(83-73-8) |
| EPA Substance Registry System | 5,7-Diiodo-8-quinolinol (83-73-8) |
Safety information for 5,7-Diiodo-8-quinolinol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. |
Computed Descriptors for 5,7-Diiodo-8-quinolinol
| InChIKey | UXZFQZANDVDGMM-UHFFFAOYSA-N |
5,7-Diiodo-8-quinolinol manufacturer
Prayag Pharmachem
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Iodoquinol CAS 83-73-8View Details
Iodoquinol CAS 83-73-8View Details
83-73-8 -
 Di-Iodohydroxyquinoline (Iodoquinol) raw materialView Details
Di-Iodohydroxyquinoline (Iodoquinol) raw materialView Details
83-73-8 -
 DiiodohydroxyquinolineView Details
DiiodohydroxyquinolineView Details
83-73-8 -
 5,7-Di Iodoquinolin-8-OlView Details
5,7-Di Iodoquinolin-8-OlView Details
83-73-8 -
 DiiodohydroxyquinolineView Details
DiiodohydroxyquinolineView Details
83-73-8 -
 DI-IODOHYDROXYQUINOLINE (IODOQUINOL)View Details
DI-IODOHYDROXYQUINOLINE (IODOQUINOL)View Details
83-73-8 -
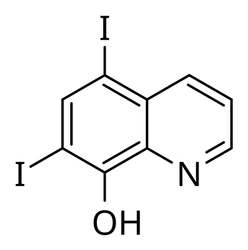 Diiodohydroxyquinoline / Iodoquinol, For Pharmaceutical, 1kg 5kg 25kg 100kg 1 MtView Details
Diiodohydroxyquinoline / Iodoquinol, For Pharmaceutical, 1kg 5kg 25kg 100kg 1 MtView Details
83-73-8 -
 Di-Iodohydroxyquinoline / IodoquinolView Details
Di-Iodohydroxyquinoline / IodoquinolView Details
83-73-8
