5,5'-DIMETHYL-2,2'-DIPYRIDYL
Synonym(s):5,5′-Dimethyl-2,2′-bipyridine
- CAS NO.:1762-34-1
- Empirical Formula: C12H12N2
- Molecular Weight: 184.24
- MDL number: MFCD01740554
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
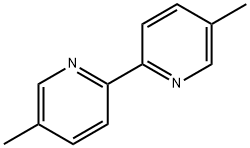
What is 5,5'-DIMETHYL-2,2'-DIPYRIDYL?
Absorption
In a pharmacokinetic trial with both adult and pediatric patients, the Cmax and AUC0-8h in the adult group were 41 ng/mL and 121 ng.h/mL and the Cmax and AUC0-8h in the pediatric group were 73 ng/mL and 264 ng.h/mL. In general, systemic exposure to abametapir appears to decrease with increasing age. The median Tmax of abemetapir is 0.57 - 1.54 hours.
Following topical administration, benzyl alcohol was found in detectable quantities in the serum of 7 out of 39 pediatric patients. The Cmax of benzyl alcohol in these subjects ranged from 0.52 to 3.57 μg/mL.
The predominant circulating metabolite of abemetapir (abemtapir carboxylate) is eliminated slowly from the circulation and is therefore found at higher serum concentrations than its parent drug - based on data collected for 72 hours post-administration, the ratios of serum Cmax and AUC0-72h between abametapir and abametapir carboxylate were approximately 30 and 250, respectively.
Toxicity
The intraperitoneal LD50 of abametapir in mice is 225 mg/kg. Topical formulations of abametapir contain benzyl alcohol, which has been associated with fatal reactions including "gasping syndrome" following systemic exposure in neonates and low birth weight infants. The use of benzyl alcohol-containing abametapir formulations should be avoided in patients <6 months of age due to an increased risk of unintentional systemic absorption. As benzyl alcohol toxicity may also occur in pediatric patients following accidental oral ingestion, the manufacturer recommends that it be administered to pediatric patients only under the direct supervision of adult. Symptoms of benzyl alcohol toxicity may include significant gastrointestinal and central nervous system adverse effects, with severe cases leading to respiratory depression and death. If toxicity is expected, patients should be advised to contact their nearest poison control center.
The minimal amount of benzyl alcohol at which toxicity might occur is unclear. Toxicity is more likely in premature infants, low birth weight infants, and those receiving high doses.
The Uses of 5,5'-DIMETHYL-2,2'-DIPYRIDYL
5,5'-Dimethyl-2,2'-bipyridine is widely used as an intermediate in organic synthesis and pharmaceuticals. It is also used as a raw material in the synthesis of dyestuffs and agrochemicals. It is used as a precursor for the preparation of 5-methyl-5?vinyl-2,2?-bipyridine.
Indications
Abametapir is indicated, in the context of an overall lice management program, for the topical treatment of head lice infestation in patients 6 months of age and older.
Background
Abametapir is a novel pediculicidal metalloproteinase inhibitor used to treat infestations of head lice. The life cycle of head lice (Pediculus capitis) is approximately 30 days, seven to twelve of which are spent as eggs laid on hair shafts near the scalp. Topical pediculicides generally lack adequate ovicidal activity, including standard-of-care treatments such as permethrin, and many require a second administration 7-10 days following the first to kill newly hatched lice that resisted the initial treatment. The necessity for follow-up treatment may lead to challenges with patient adherence, and resistance to agents like permethrin and pyrethrins/piperonyl butoxide may be significant in some areas.
Investigations into novel ovicidal treatments revealed that several metalloproteinase enzymes were critical to the egg hatching and survival of head lice, and these enzymes were therefore identified as a potential therapeutic target. Abemetapir is an inhibitor of these metalloproteinase enzymes, and the first topical pediculicide to take advantage of this novel target. The improved ovicidal activity (90-100% in vitro) of abemetapir allows for a single administration, in contrast to many other topical treatments, and its novel and relatively non-specific mechanism may help to curb the development of resistance to this agent.
Abametapir was first approved for use in the United States under the brand name Xeglyze on July 27, 2020.
Definition
ChEBI: Abametapir is a member of bipyridines.
Pharmacokinetics
Abametavir has been shown to inhibit all stages of embryo development in both head and body lice by interfering with enzymes critical to this process. It is relatively unique amongst lice treatments in that it requires only a single application, whereas many current therapies require two applications, due to its exceptional potency and unique mechanism. Its predominant metabolite, abametapir carboxyl, has a prolonged residence time in the body, with an estimated half-life of 71 ± 40 hours or longer in adults - as this metabolite has been shown to inhibit cytochrome P450 enzymes in vitro, the use of substrates of CYP3A4, CYP2B6, or CYP1A2 should be avoided for two weeks following the administration of abametapir.
Abametapir lotion is formulated with benzyl alcohol, which has been associated with significant toxicity following unintentional systemic exposure, particularly in neonates and low birth weight infants. Benzyl alcohol-containing formulations should not be administered to patients <6 months of age, and should be administered to pediatric patients cautiously and under direct supervision of an adult to mitigate the risk of unintentional oral ingestion.
Metabolism
The biotransformation of abametapir is extensive and primarily mediated by CYP1A2. It is metabolized first to abametapir hydroxyl and then further to abametapir carboxyl - the latter is cleared slowly from the plasma, resulting in higher systemic concentrations than that of the parent drug. In vitro studies suggest that abametapir carboxyl may act as an inhibitor of CYP3A4, CYP2B6, and CYP1A2, particularly at the relatively high and prolonged concentrations observed following topical administration of abametapir.
Properties of 5,5'-DIMETHYL-2,2'-DIPYRIDYL
| Melting point: | 114-117 °C(lit.) |
| Boiling point: | 140℃/3mm |
| Density | 1.060±0.06 g/cm3(Predicted) |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMF: 15mg/mL,DMSO: 10mg/mL,Ethanol: 15mg/mL,Ethanol:PBS (pH 7.2) (1:4): 0.2mg/mL |
| form | powder to crystal |
| pka | 4.78±0.32(Predicted) |
| color | White to Yellow to Orange |
| CAS DataBase Reference | 1762-34-1(CAS DataBase Reference) |
Safety information for 5,5'-DIMETHYL-2,2'-DIPYRIDYL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 5,5'-DIMETHYL-2,2'-DIPYRIDYL
| InChIKey | PTRATZCAGVBFIQ-UHFFFAOYSA-N |
5,5'-DIMETHYL-2,2'-DIPYRIDYL manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 1762-34-1 Abametapir 98%View Details
1762-34-1 Abametapir 98%View Details
1762-34-1 -
 5,5'-Dimethyl-2,2'-bipyridyl CAS 1762-34-1View Details
5,5'-Dimethyl-2,2'-bipyridyl CAS 1762-34-1View Details
1762-34-1 -
 5,5′-Dimethyl-2,2′-dipyridyl CAS 1762-34-1View Details
5,5′-Dimethyl-2,2′-dipyridyl CAS 1762-34-1View Details
1762-34-1 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
