5-Methylthiophene-2-carboxaldehyde
Synonym(s):bread thiophene;NSC 87542
- CAS NO.:13679-70-4
- Empirical Formula: C6H6OS
- Molecular Weight: 126.18
- MDL number: MFCD00005434
- EINECS: 237-178-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
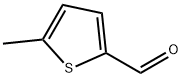
What is 5-Methylthiophene-2-carboxaldehyde?
Description
May be synthesized from thiophene (or its derivatives) and formamide in the presence of POCl3; from N-(2-thenyl)formaldimines.
Chemical properties
CLEAR YELLOW TO BROWN LIQUID
Occurrence
Reported found in French fried potato, roasted peanuts, tomato, wheat bread, raw chicken, cooked beef, pork liver, cognac, malt whiskey, coffee, popcorn, krill, shrimp and okra
The Uses of 5-Methylthiophene-2-carboxaldehyde
5-Methylthiophene-2-carboxaldehyde is used as a food additive. It is an analytical reagent. The reagent has been synthesized and characterized using IR, 1H NMR and Mass spectral data.
Preparation
From thiophene (or its derivatives) and formamide in the presence of POCl3; from N-(2-thienyl)formaldimines.
Definition
ChEBI: 5-Methyl-2-thiophenecarboxaldehyde is a member of thiophenes.
Taste threshold values
Taste characteristics at 5 ppm: sweet, almond, fruity, heliotropine and nutty
General Description
5-Methyl-2-thiophenecarboxaldehyde can be obtained as a product from 2-thiophenecarboxaldehyde via metalation, and alkylation, followed by a series of reactions.
Properties of 5-Methylthiophene-2-carboxaldehyde
| Melting point: | 184-186 °C |
| Boiling point: | 114 °C/25 mmHg (lit.) |
| Density | 1.17 g/mL at 25 °C (lit.) |
| refractive index | n |
| FEMA | 3209 | 5-METHYL-2-THIOPHENECARBOXALDEHYDE |
| Flash point: | 190 °F |
| storage temp. | 2-8°C |
| solubility | Soluble in ether and ethanol. |
| form | Liquid |
| Specific Gravity | 1.180 |
| color | Clear yellow to brown |
| Odor | at 0.10 % in dipropylene glycol. sweet almond cherry furfural woody acetophenone |
| Sensitive | Air Sensitive |
| JECFA Number | 1050 |
| BRN | 106896 |
| CAS DataBase Reference | 13679-70-4(CAS DataBase Reference) |
| NIST Chemistry Reference | 5-Methyl-2-thiophenecarboxaldehyde(13679-70-4) |
| EPA Substance Registry System | 2-Thiophenecarboxaldehyde, 5-methyl- (13679-70-4) |
Safety information for 5-Methylthiophene-2-carboxaldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H227:Flammable liquids |
| Precautionary Statement Codes |
P403+P235:Store in a well-ventilated place. Keep cool. |
Computed Descriptors for 5-Methylthiophene-2-carboxaldehyde
| InChIKey | VAUMDUIUEPIGHM-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 5-Methylthiophene-2-carboxaldehyde CAS 13679-70-4View Details
5-Methylthiophene-2-carboxaldehyde CAS 13679-70-4View Details
13679-70-4 -
 5-Methyl-2-thiophenecarboxaldehyde CAS 13679-70-4View Details
5-Methyl-2-thiophenecarboxaldehyde CAS 13679-70-4View Details
13679-70-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1