5-METHOXY-2-METHYLINDOLE
Synonym(s):NSC 63817
- CAS NO.:1076-74-0
- Empirical Formula: C10H11NO
- Molecular Weight: 161.2
- MDL number: MFCD00005620
- EINECS: 214-066-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-07-07 20:48:41
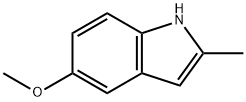
What is 5-METHOXY-2-METHYLINDOLE?
Description
5-methoxy-2-methylindole is a useful organic raw material. It can be used as the reactant in the preparation of indolylquinoxalines by condensation reactions, in preparation of alkylindoles via Ir-catalyzed reductive alkylation, in arylation reactions in the presence of a palladium acetate catalyst, in enantioselective Friedel-Crafts alkylation, as well as in stereo selective synthesis of cyclopentaindolones via stereoselective [3+2] cyclopentannulation. It is also employed in inhibiting the chlorinating activity of myeloperoxidase (MPO) and in the metabolic synthesis of acrylacetic acid anti-inflammatory synthesis.
Chemical properties
white to light beige crystalline powder or
The Uses of 5-METHOXY-2-METHYLINDOLE
An indole derivative shown to be an effective inhibitor of the chlorinating activity of myeloperoxidase (MPO). Used in the metabolic synthesis of arylacetic acid antiinflammatory synthesis.
The Uses of 5-METHOXY-2-METHYLINDOLE
reactant in preparation of indolylquinoxalines by condensation reactions1reactant in preparation of alkylindoles via Ir-catalyzed reductive alkylation2reactant in arylation reactions using a palladium acetate catalyst3reactant in enantioselective Friedel-Crafts alkylation4reactant in stereoselective synthesis of cyclopentaindolones via stereoselective [3+2] cyclopentannulation5
The Uses of 5-METHOXY-2-METHYLINDOLE
It is employed as a reactant in preparation of indolylquinoxalines by condensation reactions, reactant in preparation of alkylindoles via Ir-catalyzed reductive alkylation, reactant in arylation reactions using a palladium acetate catalyst, reactant in enantioselective Friedel-Crafts alkylation and reactant in stereoselective synthesis of cyclopentaindolones via stereoselective [3+2] cyclopentannulation. As an indole derivative shown to be an effective inhibitor of the chlorinating activity of myeloperoxidase (MPO). Used in the metabolic synthesis of arylacetic acid anti-inflammatory synthesis.
Definition
ChEBI: 5-Methoxy-2-methyl-1H-indole is a member of indoles.
References
https://www.alfa.com/en/catalog/B21348/
http://www.sigmaaldrich.com/catalog/product/aldrich/m15451?lang=en®ion=US
https://pubchem.ncbi.nlm.nih.gov/compound/5-Methoxy-2-methylindole#section=2D-Structure
Properties of 5-METHOXY-2-METHYLINDOLE
| Melting point: | 86-88 °C (lit.) |
| Boiling point: | 145 °C / 1.5mmHg |
| Density | 1.0840 (rough estimate) |
| refractive index | 1.5200 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Soluble in methanol (very faint turbidity.) |
| form | Crystalline Powder or Crystals |
| pka | 17.28±0.30(Predicted) |
| color | White to light beige |
| CAS DataBase Reference | 1076-74-0(CAS DataBase Reference) |
Safety information for 5-METHOXY-2-METHYLINDOLE
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H332:Acute toxicity,inhalation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 5-METHOXY-2-METHYLINDOLE
| InChIKey | VSWGLJOQFUMFOQ-UHFFFAOYSA-N |
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 5-Methoxy-2-methylindole CAS 1076-74-0View Details
5-Methoxy-2-methylindole CAS 1076-74-0View Details
1076-74-0 -
 5-Methoxy-2-methylindole CAS 1076-74-0View Details
5-Methoxy-2-methylindole CAS 1076-74-0View Details
1076-74-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1