5-Hydroxymethylfurfural
Synonym(s):HMF;5-Hydroxymethyl-2-furaldehyde;5-(Hydroxymethyl)furfural;5-Hydroxymethyl-2-furancarboxaldehyde;5-Hydroxymethyl-2-furancarbaldehyde
- CAS NO.:67-47-0
- Empirical Formula: C6H6O3
- Molecular Weight: 126.11
- MDL number: MFCD00003234
- EINECS: 200-654-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
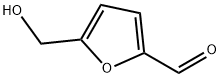
What is 5-Hydroxymethylfurfural?
Description
5-Hydroxymethylfurfural (5-HMF) derived from sugars is the link between biomass and furan-based chemicals. With its various functional groups and associated reaction sites, this small molecule opens the door to a wide range of chemical modifications, which makes 5-HMF a versatile renewable building block.
5-HMF is widely spread in food products and is formed in sugar containing food when exposed to heat. It is widely used as an indicator of quality in food products. It is also employed to indicate the adulteration of food products with acid converted invert syrups.
Description
5-(Hydroxymethyl)furfural (HMF) is derived by dehydrating sugars, fructose in particular. It was first synthesized from fructose by W. N. Haworth and W. G. M. Jones in 1944. It has been used as a starting material to synthesize compounds with oxygen-containing functional groups; HMF-derived 2,5-dimethyfuran has been evaluated as a biofuel.?Recently, HMF was shown to be toxic to honeybees fed with high-fructose corn syrup.
Chemical properties
Beige Coloured Crystalline Solid.
Among the organic compounds that can be obtained from biomass, 5-Hydroxymethylfurfural (5-HMF) is one of the most interesting ones. 5-HMF is an organic compound derived from dehydration of certain sugars (hexoses). This yellow, low-melting solid is highly water-soluble. The molecule consists of a furan ring, containing both aldehyde and alcohol functional groups. HMF as natural substance has been identified in a wide variety of heat-processed foods including milk, fruit juices, spirits, honey, etc. HMF, which is derived from cellulose, is a potential "carbon-neutral" feedstock for a number of chemical substances.
The Uses of 5-Hydroxymethylfurfural
5-Hydroxymethylfurfural is a chemical substance produced by dehydration of glucose or fructose. The molecule contains a furan ring, an aldehyde group and a hydroxymethyl group. The chemical properties are relatively lively. 5-Hydroxymethylfurfural can prepare various derivatives through oxidation, hydrogenation and condensation, and is an important fine chemical raw material.
5-Hydroxymethylfurfural is a furfural compound with a furan ring structure produced by dehydration of monosaccharide compounds such as glucose under high temperature or weak acid conditions. It is an endogenous pollutant with potential safety hazards. 5-Hydroxymethylfurfural is an important chemical raw material. Its molecule contains an aldehyde group and a hydroxymethyl group, which can be used to synthesize many useful compounds and new polymer materials through hydrogenation, oxidative dehydrogenation, esterification, halogenation, polymerization, hydrolysis and other chemical reactions, including Medicine, resin-based plastics, diesel fuel additives, etc.
The Uses of 5-Hydroxymethylfurfural
An antioxidant for grape and apple juice.
The Uses of 5-Hydroxymethylfurfural
As an indicator for excess heat-treatment in sugar-containing food.
The Uses of 5-Hydroxymethylfurfural
5-Hydroxymethyl-2-furaldehyde is used as an intermediate of OMBF (5,5'-oxydimethylenebis(2- furfural)), crown ethers. It is used in organic synthesis to prepare dialdehydes, glycols, ethers, amino alcohols and acetals. It acts as an antioxidant found in some fruit juices and caramel products.
What are the applications of Application
5-Hydroxymethyl-2-furaldehyde is an antioxidant found in some fruit juices
Definition
ChEBI: 5-hydroxymethylfurfural is a member of the class of furans that is furan which is substituted at positions 2 and 5 by formyl and hydroxymethyl substituents, respectively. Virtually absent from fresh foods, it is naturally generated in sugar-containing foods during storage, and especially by drying or cooking. It is the causative component in honey that affects the presystemic metabolism and pharmacokinetics of GZ in-vivo. It has a role as an indicator and a Maillard reaction product. It is a member of furans, an arenecarbaldehyde and a primary alcohol.
What are the applications of Application
5-Hydroxymethylfurfural has been used as a standard in the high performance liquid chromatographic (HPLC) analysis for the determination of 5-(hydroxymethyl)furfural content in co-hydrolysates and fermentation broth of lignocellulosic biomass for the lipid accumulation in M. isabellina type of microorganisms. 5-(Hydroxymethyl)furfural may be used as an analytical reference standard for the quantification of the analyte in vinegar and fruit juice samples using chromatography techniques.
5-Hydroxymethylfurfural has been used as standard in the high performance liquid chromatographic (HPLC) analysis for the determination of 5-(hydroxymethyl)furfural content in co-hydrolysates and fermentation broth of lignocellulosic biomass for the lipid accumulation in M. isabellina type of microorganisms.
Preparation
5-Hydroxymethylfurfural (HMF) was synthesized from glucose in a slug flow capillary microreactor, using a combination of AlCl3 and HCl as the homogeneous catalyst in the aqueous phase and methyl isobutyl ketone as the organic phase for in-situ HMF extraction.
Continuous synthesis of 5-hydroxymethylfurfural from glucose using a combination of AlCl3 and HCl as catalyst in a biphasic slug flow capillary microreactor
What are the applications of Application
5-Hydroxymethylfurfural can effectively prevent and treat neurodegenerative diseases, cognitive impairment and cardiovascular disease against myocardial ischemia, inhibit tumor and lower blood cholesterol. Uses 5-Hydroxymethylfurfural (5-HMF) is an important furan compound, which is widely used in medicine, chemistry, energy and other fields due to its excellent chemical properties. One of the important platform chemicals, its derivatives have great application prospects in the fields of fine chemicals, medicine, degradable plastics, etc. In particular, bio-based PEF polyester based on furandicarboxylic acid has shown better performance than petroleum-based PET (polyparaben). many properties of ethylene phthalate). 5-Hydroxymethylfurfural can be used to detect metabolites of glucose bolus infusion.
Synthesis Reference(s)
The Journal of Organic Chemistry, 59, p. 7259, 1994 DOI: 10.1021/jo00103a016
General Description
5-(Hydroxymethyl)furfural is an organic compound, widely spread in food products and is formed in sugar containing food when exposed to heat. It is widely used as an indicator of quality in food products. It is also employed to indicate the adulteration of food products with acid converted invert syrups.
Biological Activity
5-Hydroxymethylfurfural is a furanic compound derived from the degradation of sugars. It can be derived from reducing sugars via acid-catalyzed degradation or the Maillard reaction during the heating and storage of foods. 5-Hydroxymethylfurfural is an intermediate in the synthesis of a variety of compounds including 2,5-diformylfuran (DFF), 2,5-furandicarboxylic acid (FDA), 2,5-bis(hydroxymethyl)furan (5-(hydroxymethyl)furfuryl alcohol; Item No. 20658), and dimethylfuran (DMF), among others. 5-Hydroxymethylfurfural has been found in the marine algae L. undulata and scavenges 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydroxyl, alkyl, and superoxide radicals in cell-free assays (IC50s = 27.1, 22.8, 45, and 33.5 μM, respectively).
Safety Profile
Questionable carcinogen with experimental tumorigenic data.Mutation data reported. When heated to decomposition it emits acrid smoke and irritating fumes. See also ALDEHYDES
Safety
5-Hydroxymethylfurfural is irritating to eyes, mucous membranes and skin, and has neurotoxicity and genotoxicity; it can be metabolized into 5-sulfoxymethylfurfural (SMF) in vivo, which has strong carcinogenicity and Genotoxicity. Chemical properties Light yellow waxy, easily soluble in methanol and ethanol, derived from Cornus, with high content of d-glucose. Uses for content determination/identification/pharmacological experiments, etc.
Metabolic pathway
When 5-hydroxymethyl-2-furaldehyde (HMF) is administered orally or intravenously to rats, HMF or its metabolites are rapidly eliminated in the urine with the recovery of 95 ? 100% after 24 h. HMF is completely converted to two metabolites which are identified as 5- hydroxymethyl-2-furoic acid and N-(5-hydroxymethyl-2- furoyl)glycine.
Purification Methods
Crystallise it from diethyl ether/pet ether. [Beilstein 18 III/IV 100, 18/1 V 130.]
Toxicity evaluation
5-Hydroxymethylfurfural (5-HMF) as a product of the Maillard reaction is found in many foods. Estimated intakes range between 4 and 30?mg per person and day, while an intake of up to 350?mg can result from, e.g., beverages made from dried plums. In vitro genotoxicity was positive when the metabolic preconditions for the formation of the reactive metabolite 5-sulphoxymethylfurfural were met. However, so far in vivo genotoxicity was negative. Results obtained in short-term model studies for 5-HMF on the induction of neoplastic changes in the intestinal tract were negative or cannot be reliably interpreted as “carcinogenic”. In the only long-term carcinogenicity study in rats and mice no tumours or their precursory stages were induced by 5-HMF aside from liver adenomas in female mice, the relevance of which must be viewed as doubtful. Hence, no relevance for humans concerning carcinogenic and genotoxic effects can be derived. The remaining toxic potential is rather low. Various animal experiments reveal that no adverse effect levels are in the range of 80–100?mg/kg body weight and day. Safety margins are generally sufficient. However, 5-HMF exposure resulting from caramel colours used as food additives should be further evaluated.
Toxicology and risk assessment of 5-Hydroxymethylfurfural in food
Properties of 5-Hydroxymethylfurfural
| Melting point: | 28-34 °C (lit.)
28-34 °C (lit.) |
| Boiling point: | 114-116 °C/1 mmHg (lit.) |
| Density | 1.243 g/mL at 25 °C (lit.) |
| refractive index | n |
| Flash point: | 175 °F |
| storage temp. | 2-8°C |
| solubility | Chloroform (Slightly), Ethyl Acetate (Slightly), Methanol (Slightly) |
| form | Liquid or Crystalline Powder and/or Chunks |
| pka | 12.82±0.10(Predicted) |
| color | Light yellow to yellow |
| Odor | at 0.10 % in dipropylene glycol. fatty buttery musty waxy caramellic |
| Water Solubility | Soluble in water, alcohol, ethyl acetate, acetone, dimethylformamide, benzene, ether and chloroform. |
| Sensitive | Air & Light Sensitive |
| Merck | 14,4832 |
| BRN | 110889 |
| Stability: | Light Sensitive, Very Hygroscopic |
| CAS DataBase Reference | 67-47-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Furancarboxaldehyde, 5-(hydroxymethyl)-(67-47-0) |
| EPA Substance Registry System | 2-Furancarboxaldehyde, 5-(hydroxymethyl)- (67-47-0) |
Safety information for 5-Hydroxymethylfurfural
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for 5-Hydroxymethylfurfural
| InChIKey | NOEGNKMFWQHSLB-UHFFFAOYSA-N |
5-Hydroxymethylfurfural manufacturer
Neugen Labs
Anand Agencies
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran





You may like
-
 5-Hydroxymethylfurfural (HMF) ExiPlus CAS 67-47-0View Details
5-Hydroxymethylfurfural (HMF) ExiPlus CAS 67-47-0View Details
67-47-0 -
 5-Hydroxymethyl-2-furaldehyde (stabilized with Water) CAS 67-47-0View Details
5-Hydroxymethyl-2-furaldehyde (stabilized with Water) CAS 67-47-0View Details
67-47-0 -
 5-Hydroxymethylfurfural (HMF) extrapure CAS 67-47-0View Details
5-Hydroxymethylfurfural (HMF) extrapure CAS 67-47-0View Details
67-47-0 -
 5-Hydroxymethyl furfural, 98% CAS 67-47-0View Details
5-Hydroxymethyl furfural, 98% CAS 67-47-0View Details
67-47-0 -
 5-Hydroxymethyl-2-furaldehyde CAS 67-47-0View Details
5-Hydroxymethyl-2-furaldehyde CAS 67-47-0View Details
67-47-0 -
 5-Hydroxymethyl-2-furancarbaldehyde CAS 67-47-0View Details
5-Hydroxymethyl-2-furancarbaldehyde CAS 67-47-0View Details
67-47-0 -
 Glucosamine Impurity E CAS 67-47-0View Details
Glucosamine Impurity E CAS 67-47-0View Details
67-47-0 -
 5-(Hydroxymethyl)furfural CAS 67-47-0View Details
5-(Hydroxymethyl)furfural CAS 67-47-0View Details
67-47-0