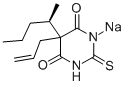
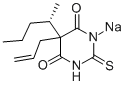
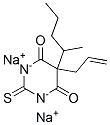
5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID
Synonym(s):5-Allyl-5-(1-methylbutyl)-2-thiobarbituric acid
- CAS NO.:77-27-0
- Empirical Formula: C12H18N2O2S
- Molecular Weight: 254.35
- MDL number: MFCD00057565
- EINECS: 201-018-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-19 15:37:50
![5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID Structural](https://img.chemicalbook.in/CAS/GIF/77-27-0.gif)
What is 5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID?
Absorption
Rapidly absorbed (high lipid solubility).
Toxicity
Intravenous LD50 in rat is 51 mg/kg.
Description
Thiamylal, 5-allyl-5-(1- methylbutyl)-2-thiobarbituric acid, The sodium salt , Mr 276.36, is an amorphous powder soluble in water. It is prepared by condensation of thiourea with a malonic ester , .
Originator
Surital,Parke Davis,US,1951
The Uses of 5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID
Thiamylal is a barbiturate derivative with sedative, anticonvulsant and hypnotic effects. Thiamylal is used for induction in surgical anaesthesia as well as an anticonvulsant to counteract side effects from other anaesthetics. Thiamylal is a Controlled Substance.
Indications
Used for the production of complete anaesthesia of short duration, for the induction of general anaesthesia, and for inducing a hypnotic state.
Background
A barbiturate that is administered intravenously for the production of complete anesthesia of short duration, for the induction of general anesthesia, or for inducing a hypnotic state. (From Martindale, The Extra Pharmacopoeia, 30th ed, p919)
Definition
ChEBI: A member of the class of barbiturates that is 2-thioxodihydropyrimidine-4,6(1H,5H)-dione substituted by a pentan-2-yl and prop-2-en-1-yl group at position 5.
Manufacturing Process
In 450 cc of methanol is added 47 grams of sodium metal and the mixture
allowed to completely react to form a methanol solution of sodium methoxide.
The methanol solution of sodium methoxide is then cooled to 60°C and 68
grams of thiourea which has been thoroughly dried is added with stirring until
a uniform solution is formed. Thereafter, 157 grams of diethyl allyl-(1-
methylbutyl)malonate is added to the solution of the sodio derivative of
thiourea at a temperature of 55°C and the condensation reaction mixture
maintained at the said temperature for 24 hours. Methyl alcohol is removed
under vacuum during the course of the reaction while maintaining a
temperature of 55°C.
The viscous reaction mixture is then poured into 1.5 liters of ice water and
agitated to form a uniform solution. The solution is treated with activated
carbon and filtered. Thereafter, 80% acetic acid is added until the filtered
solution remains acidic to litmus. The precipitate formed is filtered and
washed thoroughly with distilled water. The product is air-dried at a temperature of 95° to 100°C for 48 hours to yield 133 grams of 5-allyl-5-(1-
methylbutyl)-2-thiobarbituric acid having a melting point of 132° to 133°C
and assaying at 99.5% pure, from US Patent 2,876,225.
Therapeutic Function
Anesthetic
Pharmacokinetics
Thiamylal, a barbiturate, is used in combination with acetaminophen or aspirin and caffeine for its sedative and relaxant effects in the treatment of tension headaches, migraines, and pain. Barbiturates act as nonselective depressants of the central nervous system (CNS), capable of producing all levels of CNS mood alteration from excitation to mild sedation, hypnosis, and deep coma. In sufficiently high therapeutic doses, barbiturates induce anesthesia.
Metabolism
Hepatic.
Properties of 5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID
| Melting point: | 132-133° |
| Density | 1.1828 (rough estimate) |
| refractive index | 1.5510 (estimate) |
| pka | 7.01±0.40(Predicted) |
| form | neat |
| Water Solubility | 1.298g/L(25 ºC) |
Safety information for 5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for 5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran
![5-ALLYL-5-[1-METHYLBUTYL]-2-THIOBARBITURIC ACID](https://img.chemicalbook.in/CAS/GIF/77-27-0.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8