4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
Synonym(s):2,2-Bis(3,4-dicarboxyphenyl)-1,1,1,3,3,3-hexafluoropropane;4,4′-(Hexafluoroisopropylidene)bis(phthalic anhydride);4,4′-(Hexafluoroisopropylidene)diphthalic anhydride
- CAS NO.:1107-00-2
- Empirical Formula: C19H6F6O6
- Molecular Weight: 444.24
- MDL number: MFCD00039143
- EINECS: 214-170-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
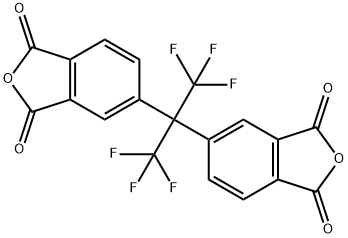
What is 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride?
Description
4,4′-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) is an aromatic organofluorine compound and the dianhydride of 4,4'-(hexafluorisopropylidene)bisphthalic acid (name derived from phthalic acid). It is one of the six most widely used dianhydride monomers, with good balance of mechanical properties and electrical properties, and it is also the most widely used dianhydride monomer in colorless and transparent polyimides.
Physical properties
Beige crystal
The Uses of 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
4,4'-(hexafluoroisopropylidene)diphthalic anhydride be used for the preparation of a polyimide material.
The Uses of 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
4,4'-(Hexafluoroisopropylidene) diphthalic anhydride is involved in the synthesis of polyamides derived from terphenylenes, which is used for gas separation.
General Description
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride (6FDA) consists of two phthalic anhydrides bridged with a perfluoroisopropyl group and is mainly used in the synthesis of polyimides. Polyimides from 6FDA are commonly used in hybrid matrix membranes. The polyimide matrix provides good dispersion of the filler and can be loaded with up to 50 wt% filler. These membranes have been used for highly selective gas separation (CO2/CH4) and solar cell encapsulation (power conversion efficiency of silicon-based solar cells increased from 14.67% to 15.64%).
Advantages
The 4,4'-(hexafluoroisopropylidene) diphthalic anhydride (6FDA) monomer contains rigid benzene ring groups, and so, the resulting polymer has excellent mechanical properties. 6FDA contained a stable benzene ring and fluorine atoms. Due to the strong electronegativity of the fluorine atoms and the stability of the benzene ring, the produced polycarbonate had better thermal stability[1-2].
Precautions
The storage precautions for 6FDA are as follows: The chemical must be stored in a cool and well-ventilated warehouse, away from sources of heat and fire. Proper sealing is required to prevent contact with air, and it should always be kept separate from oxidants, edible chemicals, and acids. The storage area should have explosion-proof lighting and ventilation facilities and must not allow any mechanical equipment or tools that may cause sparks to prevent accidents. Leakage emergency treatment equipment and suitable storage materials must be readily available in the storage area.
References
[1] Yile Zhang. “Poly(propylene carbonate) networks with excellent properties: Terpolymerization of carbon dioxide, propylene oxide, and 4,4?-(hexafluoroisopropylidene) diphthalic anhydride.” e-Polymers 21 1 (2021): 511–519.
[2] C. D. Simone, D. A. Scola. “Phenylethynyl End-Capped Polyimides Derived from 4,4‘-(2,2,2-Trifluoro-1-phenylethylidene)diphthalic Anhydride, 4,4‘-(Hexafluoroisopropylidene)diphthalic Anhydride, and 3,3‘,4,4‘-Biphenylene Dianhydride: Structure?Viscosity Relationship.” Macromolecules 36 18 (2003): 6780–6790.
[3] MARYANNE MORES Patrick E C. Polyimidines. XI. Polyimidines from 3,3′,4,4′-benzophenonetetracarboxylic dianhydride and 4,4′-(hexafluoroisopropylidene) diphthalic anhydride[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1990, 28 4: 811-823. DOI:10.1002/pola.1990.080280410.
[4] HUI WU Hezhou L Hua Li. Synthesis and Properties of a High-Molecular-Weight Polyimide Based on 4, 4’-(hexafluoroisopropylidene) Diphthalic Anhydride[J]. Advanced Materials Research, 2012, 35 1: 742-746. DOI:10.4028/www.scientific.net/AMR.550-553.742.
Properties of 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
| Melting point: | 244-247 °C(lit.) |
| Boiling point: | 494.5±45.0 °C(Predicted) |
| Density | 1.697±0.06 g/cm3(Predicted) |
| vapor pressure | 7.7Pa at 20℃ |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| form | powder to crystal |
| color | White to Almost white |
| Water Solubility | Miscible with water. |
| Sensitive | Moisture Sensitive |
| BRN | 7057916 |
| InChI | InChI=1S/C19H6F6O6/c20-18(21,22)17(19(23,24)25,7-1-3-9-11(5-7)15(28)30-13(9)26)8-2-4-10-12(6-8)16(29)31-14(10)27/h1-6H |
| CAS DataBase Reference | 1107-00-2(CAS DataBase Reference) |
| EPA Substance Registry System | 1,3-Isobenzofurandione, 5,5'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]bis- (1107-00-2) |
Safety information for 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
| InChIKey | QHHKLPCQTTWFSS-UHFFFAOYSA-N |
| SMILES | C(C1C=CC2C(=O)OC(=O)C=2C=1)(C1C=CC2C(=O)OC(=O)C=2C=1)(C(F)(F)F)C(F)(F)F |
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid Fmoc-Val-Cit-PAB DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonateRelated products of tetrahydrofuran

You may like
-
 4,4'-(Hexafluoroisopropylidene)diphthalic anhydride CAS 1107-00-2View Details
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride CAS 1107-00-2View Details
1107-00-2 -
 4,4'-(Hexafluoroisopropylidene)diphthalic Anhydride (purified by sublimation) CAS 1107-00-2View Details
4,4'-(Hexafluoroisopropylidene)diphthalic Anhydride (purified by sublimation) CAS 1107-00-2View Details
1107-00-2 -
 4,4'-(Hexafluoroisopropylidene)diphthalic Anhydride CAS 1107-00-2View Details
4,4'-(Hexafluoroisopropylidene)diphthalic Anhydride CAS 1107-00-2View Details
1107-00-2 -
 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride CAS 1107-00-2View Details
4,4′-(Hexafluoroisopropylidene)diphthalic anhydride CAS 1107-00-2View Details
1107-00-2 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8