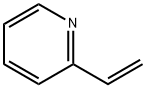
4-Vinylpyridine
- CAS NO.:100-43-6
- Empirical Formula: C7H7N
- Molecular Weight: 105.14
- MDL number: MFCD00006447
- EINECS: 202-852-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
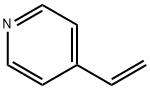
What is 4-Vinylpyridine?
Description
4-vinylpyridine (4VP) is a pyridine derivative with a vinyl group in the 4-position. It is a colorless liquid, although impure samples are often brown. This compound belongs to a class of polar vinyl monomers that are important in many applications due to the ability of their polymers to form complexes of the electron-rich pyridine ring and to form polyelectrolytes through protonation or quaternization with alkyl or aryl halides. 4VP is sometimes used in biochemistry to alkylate protein cysteine residues. When compared to other alkylation agents, such as iodoacetamide, acrylamide, and N-ethylmaleimide, 4VP is less reactive, meaning the completion rate of cysteine alkylation is lower, but it also yields fewer side reactions[1].
Chemical properties
dark brown to yellow liquid. 2.9g can be dissolved in 100g of water at 20℃, soluble in methanol, ether, benzene, acetone, and slightly soluble in ether. When exposed to light and heat, polymerization occurs, and 0.1%-0.2% hydroquinone is often added as a polymerization inhibitor during storage.
The Uses of 4-Vinylpyridine
4-Vinylpyridine acts as a co-monomer in styrene-butadiene polymers to promote adhesion between the rubber compound and supporting fibers (or cords) in tires, belts and hoses. It serves as an intermediate for manufacturing chemicals and pharmaceuticals. Further, it is used as a reagent for the modification of cysteine. In addition to this, it is used to make poly(4-vinylpyridine) and elastomers.
What are the applications of Application
4-Vinylpyridine was used as a monomer in polymer chemistry and induced nonimmunological contact urticaria and allergic contact dermatitis. No crossreactivity is observed between pyridine derivatives. It can be copolymerized with many other monomers, in particular cationic monomers, such as acrylamide, acrylates, and also with other vinyl-based monomers (e.g. styrene).?
Preparation
4-Vinylpyridine is synthesized by reacting 4-picoline with formaldehyde to generate 4-pyridineethanol, which is then dehydrated.
Flammability and Explosibility
Flammable
Purification Methods
6 2 . Purify the monomer by fractional distillation under a good vacuum and in a N2 atmosphere and store it in sealed ampoules under N2, and keep it in the dark at -20o. The picrate has m 175-176o. [UV: Coleman & Fuoss J Am Chem Soc 77 5472 1955, Overberger et al. J Polymer Sci 27 381 1958, Petro & Smyth J Am Chem Soc 79 6142 1957.] It is used for alkylating SH groups in peptides [Anderson & Friedman Can J Biochem 49 1042 1971, Cawins & Friedman Anal Biochem 35 489 1970]. [Beilstein 20 II 170, 20 III/IV 2887, 20/6 V 213.]
References
[1] Baskaran, D. and A. Müller. “Anionic Polymerization of Polar Vinyl Monomers.” (2012): 623-655
Properties of 4-Vinylpyridine
| Melting point: | <25 °C |
| Boiling point: | 62-65 °C/15 mmHg (lit.) |
| Density | 0.975 g/mL at 25 °C (lit.) |
| vapor pressure | 2.28hPa at 25℃ |
| refractive index | n |
| Flash point: | 125 °F |
| storage temp. | -20°C |
| solubility | Chloroform, Methanol (Slightly) |
| form | Liquid |
| pka | pK1:5.62(+1) (25°C) |
| color | Clear colorless to yellow-brown |
| Water Solubility | 29 G/L (20 ºC) |
| BRN | 104506 |
| Exposure limits | ACGIH: TWA 1 mg/m3 OSHA: TWA 2 mg/m3 NIOSH: IDLH 50 mg/m3; Ceiling 2 mg/m3 |
| Stability: | Stable. Incompatible with strong acids, strong oxidizing agents. |
| CAS DataBase Reference | 100-43-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Pyridine, 4-ethenyl-(100-43-6) |
| EPA Substance Registry System | 4-Vinylpyridine (100-43-6) |
Safety information for 4-Vinylpyridine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H314:Skin corrosion/irritation H317:Sensitisation, Skin H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Vinylpyridine
New Products
3-Iodophenylacetic acid 3-Pyridineacetonitrile, α-hydroxy- 2-Propanamine, 1-chloro-, hydrochloride (9CI) 3-(hexyloxy)-4-(pyridin-3-yl)-1,2,5-thiadiazole 2-Hexyn-1-ol Dibenzo-18-crown-6 Nickel(II) perchlorate hexahydrate, 98% 4-Bromophenylacetonitrile, 95% 3-Bromo-4-fluoroaniline, 97% Sodium tetraborate decahydrate, 98% Palladium(II) acetate, trimer, Pd 99% 4-Bromo-2-chlorotoluene, 97% N N Dimethylformamide Dimethyl Acetal (Dmf Dma) 2,3-Dichloro Benzoyl Cyanide [Side Chain] Bis(2-Chloroethyl) Amine Hydrochloride L-Glutamic Acid Diethyl Ester Hydrochloride 5-(Difluoromethoxy)-2-Mercaptobenzimidazole 1-Ethyl-3-(3-Dimethylaminopropyl)-Carbodiimide Hydrochloride [EDC Hcl] 1,4-Napthoquinone Bromoiodomethane Sodium Bicarbonate Methylene Dichloride (MDC) Ethyl Acetate Indole-3-Carbinol (I3C)Related products of tetrahydrofuran



You may like
-
 4-Vinylpyridine, stabilized with 100ppm hydroquinone CAS 100-43-6View Details
4-Vinylpyridine, stabilized with 100ppm hydroquinone CAS 100-43-6View Details
100-43-6 -
 4-Vinylpyridine (stabilized with HQ) CAS 100-43-6View Details
4-Vinylpyridine (stabilized with HQ) CAS 100-43-6View Details
100-43-6 -
 4-Vinylpyridine 95% CAS 100-43-6View Details
4-Vinylpyridine 95% CAS 100-43-6View Details
100-43-6 -
 4-Vinylpyridine CAS 100-43-6View Details
4-Vinylpyridine CAS 100-43-6View Details
100-43-6 -
 17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 3-Pyridineacetonitrile, α-hydroxy- 98+View Details
17604-74-9 -
 Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
Cyclohexane, (2-propynyloxy)- 67967-07-1 98+View Details
67967-07-1 -
 3-Iodophenylacetic acid 1878-69-9 98+View Details
3-Iodophenylacetic acid 1878-69-9 98+View Details
1878-69-9 -
 132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6 (S)-1-Boc-3-methanesulfonyloxy-pyrrolidine 98+View Details
132945-75-6
