4-tert-Butoxystyrene
Synonym(s):4-tert -Butoxystyrene;p -tert -Butoxystyrene
- CAS NO.:95418-58-9
- Empirical Formula: C12H16O
- Molecular Weight: 176.25
- MDL number: MFCD00145183
- EINECS: 619-127-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-01-27 09:38:02
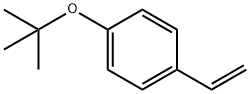
What is 4-tert-Butoxystyrene?
Description
4-tert-butoxystyrene is a pendant functionalized styrene that structurally, It is similar to PMOS, both carrying an alkoxyl p-substituent, whose electron-donating and resonance effects allow them to be'polymerized by cationic, anionic, and radical mechanisms. It is used to treatment of its polymers with an acid leads to poly(4-vinylphenol), which finds a wide variety of applications to photoresists, epoxy-curing agents, adhesives, etc.).
Chemical properties
Colorless to Almost colorless clear liquid.
The Uses of 4-tert-Butoxystyrene
4-tert-Butoxystyrene is used in organic synthesis. It is used to synthesize 4'-tert-butoxy-biphenyl-4-carboxylic acid methyl ester.
What are the applications of Application
4-tert-Butoxystyrene is an organic synthetic reagent used as a polymer monomer in the preparation of poly(4-tert-butoxystyrene) (PtBSt) and other living polymers. Poly(4-tert-Butoxystyrene) is a new hexane-soluble polymer surfactant that can be used to form micelles.
Synthesis
Preparation of 4-tert-Butoxystyrene (3-tert-Butoxystyrene). To a 2000-mL three-necked round-bottom flask equipped with a dropping funnel, thermometer, reflux condenser, paddle stirrer and nitrogen inlet were placed 19.4 g (0.80 mol) of magnesium turnings and enough freshly dried and distilled tetrahydrofuran (THF) to cover the turnings. There were then added dropwise with stirring a solution of 143.3g (0.78 mol) of freshly distilled 4-bromostyrene (bp 46-47°C (0.03 mm)) in 500mL of THF. After 20mL of the 4-bromostyrene had been added, an exothermic reaction set in and was maintained between 25 and 35°C by adjusting the rate of addition of the bromo compound and with the aid of an ice bath. After the addition had been completed, the reaction mixture was heated to 60°C for 0.5h. Using a NaC1-ice bath, the mixture was cooled to 0°C and a solution of 100.88g (0.52 mol) of tert-butyl peroxybenzoate in 200 mL of THF was added via the dropping funnel at such a rate that the reaction temperature was maintained between 0 and 5°C. After completion of the addition, the reaction mixture was stirred at 25°C for 2 h. The organic layer was separated from the solid magnesium benzoate by decantation and the volume of the solution reduced on a rotary evaporator. The yellow oil that remained was washed with 1000 mL of 3% aqueous HCl solution and the organic layer separated. The aqueous layer was washed with two 200-mL portions of ether, and the ether and organic layers were combined and together washed with two 75-mL portions of a 10% NaOH solution followed by washing with water until the aqueous washings were neutral. After the solution was dried over Na2S04 and the ether was removed via a rotary evaporator, the remaining pale yellow oil was purified by fractional distillation in the presence of a few milligrams of ionol (2,6-ditert-butyl-4-methylphenol) as an inhibitor. There were obtained 46 g (50% yield, bp 45°C (0.02 mm)) of 4-tert-Butoxystyrene having the following elemental analysis.
References
Living cationic sequential block copolymerization of isobutylene with 4-tert-butoxystyrene: synthesis and characterization of poly(p-hydroxystyrene-b-isobutylene-b-p-hydroxystyrene) triblock copolymers.
Bouchékif, H.; Som, A.; Sipos, L.; Faust, R.; Journal of Macromolecular Science, Part A: Pure and Applied Chemistry (2007), 44(4), 359-366.
Properties of 4-tert-Butoxystyrene
| Melting point: | −38 °C(lit.) |
| Boiling point: | 72-73 °C0.1 mm Hg(lit.) |
| Density | 0.936 g/mL at 25 °C(lit.) |
| vapor pressure | 17.999Pa at 20℃ |
| refractive index | n |
| Flash point: | 207 °F |
| InChI | InChI=1S/C12H16O/c1-5-10-6-8-11(9-7-10)13-12(2,3)4/h5-9H,1H2,2-4H3 |
| CAS DataBase Reference | 95418-58-9(CAS DataBase Reference) |
Safety information for 4-tert-Butoxystyrene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-tert-Butoxystyrene
| InChIKey | GRFNSWBVXHLTCI-UHFFFAOYSA-N |
| SMILES | C1(OC(C)(C)C)=CC=C(C=C)C=C1 |
New Products
1-Amino-1-cyclohexanecarboxylic acid Cycloleucine 6-Bromo-3-iodo-1-methyl-1H-indazole 3-(2,4-Dimethoxybenzyl)dihydropyrimidine-2,4(1H,3H)-dione 7-Bromo-1H-indazole ELECTROLYTIC IRON POWDER 2-Methyl-2-phenylpropyl acetate 1-Aminocyclobutanecarboxylic acid 1-(2-Ethoxyethyl)-2-(piperidin-4-yl)-1H-benzo[d]imidazole hydrochloride Decanonitrile tert-butyl 4-(1H-benzo[d]iMidazol-2-yl)piperidine-1-carboxylate 4-Ethylbenzylamine Methyl 5-bromo-2-chloro-3-nitrobenzoate N-(5-Amino-2-methylphenyl)acetamide 2-Chloro-3-nitropyridine 5-Bromo-2,3-dimethoxypyridine methyl 6-chloro-2-(chloromethyl)nicotinate 2-methoxy-4-methyl-5-nitro pyridine 2-iodo-5-bromo pyridine 2-amino-4-methyl-5-nitro pyridine 5-Fluoro-2-Oxindole methyl L-alaninate hydrochloride diethyl L-glutamate hydrochloride Ethyl tosylcarbamateRelated products of tetrahydrofuran






You may like
-
 95418-58-9 4-tert-Butoxystyrene 99%View Details
95418-58-9 4-tert-Butoxystyrene 99%View Details
95418-58-9 -
 4-tert-Butoxystyrene CAS 95418-58-9View Details
4-tert-Butoxystyrene CAS 95418-58-9View Details
95418-58-9 -
 Ethoxymethylenemalononitrile 99% HPLCView Details
Ethoxymethylenemalononitrile 99% HPLCView Details
123-06-8 -
 Diethyl Disulfide 99% HPLCView Details
Diethyl Disulfide 99% HPLCView Details
110-81-6 -
 6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. 1-(4-chlorophenyl) propan-1-one 98%View Details
6285-05-8. -
 4-chloro-3,5-dinitropyridine 98%View Details
4-chloro-3,5-dinitropyridine 98%View Details -
 401-95-6 99% HPLCView Details
401-95-6 99% HPLCView Details
401-95-6 -
 171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1 tert-Butyl 3-bromobenzylcarbamate 98%View Details
171663-13-1