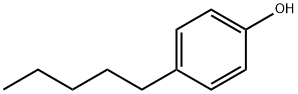
4-tert-Amylphenol
- CAS NO.:80-46-6
- Empirical Formula: C11H16O
- Molecular Weight: 164.24
- MDL number: MFCD00002369
- EINECS: 201-280-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
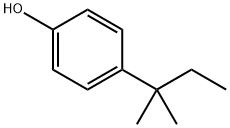
What is 4-tert-Amylphenol?
Chemical properties
solid
The Uses of 4-tert-Amylphenol
4-tert-Amylphenol was used as an esterogen receptor (ER) ligand.
The Uses of 4-tert-Amylphenol
In the manufacture of oil-soluble resins; has been recommended as a germicide and fumigant; intermediate for organic mercury germicides, for pesticides, for chemicals used in rubber and petroleum industries.
The Uses of 4-tert-Amylphenol
Demulsifiers, Biocides, Fragrances
Definition
ChEBI: P-tert-Amylphenol is an alkylbenzene.
General Description
Colorless needles or beige solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenols, such as 4-tert-Amylphenol, do not behave as organic alcohols, as one might guess from the presence of a hydroxyl (-OH) group in their structure. Instead, they react as weak organic acids. Phenols and cresols are much weaker as acids than common carboxylic acids (phenol has Ka = 1.3 x 10^[-10]). These materials are incompatible with strong reducing substances such as hydrides, nitrides, alkali metals, and sulfides. Flammable gas (H2) is often generated, and the heat of the reaction may ignite the gas. Heat is also generated by the acid-base reaction between phenols and bases. Such heating may initiate polymerization of the organic compound. Phenols are sulfonated very readily (for example, by concentrated sulfuric acid at room temperature). The reactions generate heat. Phenols are also nitrated very rapidly, even by dilute nitric acid. Nitrated phenols often explode when heated. Many of them form metal salts that tend toward detonation by rather mild shock. 4-tert-Amylphenol can react with oxidizing materials.
Health Hazard
ACUTE/CHRONIC HAZARDS: 4-tert-Amylphenol is toxic by ingestion and can be absorbed through the skin. Hazardous fumes are evolved when 4-tert-Amylphenol is heated to decomposition.
Fire Hazard
4-tert-Amylphenol is combustible.
Flammability and Explosibility
Flammable
Safety Profile
Moderately toxic by ingestion andskin contact. A skin and severe eye irritant. Combustible.When heated to decomposition it emits toxic fumes. Tofight fire, use dry chemical, water mist, CO2. Incompatiblewith oxidizing materials.
Purification Methods
Purify via its benzoate, as for phenol. After evaporating the solvent from its solution in ether, the material is recrystallised (from the melt) to a constant melting point. The benzoyl derivative has m 60o (from EtOH). [Berliner et al. J Am Chem Soc 76 507 1954, Huston et al. J Am Chem Soc 67 899 1945, Beilstein 6 H 548, 6 I 269, 6 II 506, 6 III 1965, 6 IV 3383.]
Properties of 4-tert-Amylphenol
| Melting point: | 88-89 °C (lit.) |
| Boiling point: | 255 °C (lit.) |
| Density | 0,96 g/cm3 |
| vapor pressure | 5Pa at 20℃ |
| refractive index | 1.5061 (estimate) |
| Flash point: | 111 °C |
| storage temp. | 2-8°C |
| solubility | H2O: insoluble |
| form | Briquettes or Flakes To Coarse Powder |
| pka | 10.24±0.26(Predicted) |
| color | White to pale yellow |
| Water Solubility | 37 mg/L (20 ºC) |
| Merck | 14,7142 |
| Stability: | Stable. Incompatible with acid chlorides, acid anhydrides, strong oxidizing agents. |
| CAS DataBase Reference | 80-46-6(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenol, 4-(1,1-dimethylpropyl)-(80-46-6) |
| EPA Substance Registry System | p-tert-Amylphenol (80-46-6) |
Safety information for 4-tert-Amylphenol
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H317:Sensitisation, Skin H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-tert-Amylphenol
| InChIKey | NRZWYNLTFLDQQX-UHFFFAOYSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 80-46-6 4-tert-Amylphenol 99%View Details
80-46-6 4-tert-Amylphenol 99%View Details
80-46-6 -
 4-tert-Amylphenol CAS 80-46-6View Details
4-tert-Amylphenol CAS 80-46-6View Details
80-46-6 -
 4-tert-Amylphenol, 99% CAS 80-46-6View Details
4-tert-Amylphenol, 99% CAS 80-46-6View Details
80-46-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
