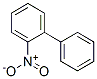
4-Nitrobiphenyl
- CAS NO.:92-93-3
- Empirical Formula: C12H9NO2
- Molecular Weight: 199.21
- MDL number: MFCD00007342
- EINECS: 202-204-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
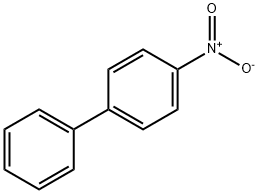
What is 4-Nitrobiphenyl?
Chemical properties
Yellow Solid
Chemical properties
4-Nitrobiphenyl exists as yellow plates or needles..
The Uses of 4-Nitrobiphenyl
4-Nitrobiphenyl has some uses as a plasticizer, fungicide, and wood preservative.
The Uses of 4-Nitrobiphenyl
Formerly in preparation of p-biphenylamine, q.v.
The Uses of 4-Nitrobiphenyl
Formerly used as an intermediate for 4-aminobiphenyl
Background
Pnb has been used in trials studying the treatment of Pelvic Organ Prolapse.
Definition
ChEBI: 4-Nitrobiphenyl is a member of biphenyls.
Synthesis Reference(s)
Tetrahedron Letters, 37, p. 4499, 1996 DOI: 10.1016/0040-4039(96)00924-0
General Description
White to yellow needle-like crystalline solid with a sweetish odor.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
4-Nitrobiphenyl is incompatible with the following: Strong reducers .
Hazard
Toxic by ingestion and skin contact. Confirmed carcinogen.
Health Hazard
p-Nitrobiphenyl (PNB) is a urinary bladder carcinogen in dogs.
Safety Profile
Confirmed carcinogen with experimental carcinogenic, neoplastigenic, and tumorigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also NITRO COMPOUNDS OF AROMATIC HYDROCARBONS.
Potential Exposure
4-Nitrobiphenyl was formerly used in the synthesis of 4-aminodiphenyl. It is presently used only for research purposes; there are no commercial uses.
Carcinogenicity
The case for the carcinogenicity of PNB is supported by (1) the induction of urinary bladder cancer in dogs after administration of PNB; (2) the evidence that PNB is metabolized in vivo to 4-aminobiphenyl (a potent carcinogen); and (3) the possibility that the cases of human urinary bladder cancer attributed to 4- aminobiphenyl may also have been induced by exposure to PNB.1
Metabolism
Not Available
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Incompatible with strong reducing agents such as hydrides, oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Waste Disposal
Incineration @ 982℃/2.0 seconds (minimum) with scrubbing for nitrogen oxides abatement.
Properties of 4-Nitrobiphenyl
| Melting point: | 114 °C |
| Boiling point: | 340 °C |
| Density | 1.1919 (rough estimate) |
| refractive index | 1.5880 (estimate) |
| Flash point: | 43 °C |
| solubility | Soluble in acetic acid, benzene, chloroform, and ether (Weast, 1986) |
| form | Solid |
| color | White |
| Water Solubility | insoluble |
| Stability: | Stable. Incompatible with strong oxidizing agents. |
| InChI | InChI=1S/C12H9NO2/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H |
| CAS DataBase Reference | 92-93-3(CAS DataBase Reference) |
| IARC | 3 (Vol. 4, Sup 7) 1987 |
| NIST Chemistry Reference | 1,1'-Biphenyl, 4-nitro-(92-93-3) |
| EPA Substance Registry System | 4-Nitrobiphenyl (92-93-3) |
Safety information for 4-Nitrobiphenyl
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H350:Carcinogenicity H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 4-Nitrobiphenyl
| InChIKey | BAJQRLZAPXASRD-UHFFFAOYSA-N |
| SMILES | C1(C2=CC=CC=C2)=CC=C([N+]([O-])=O)C=C1 |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 P-Nitrobiphenyl 97% CAS 92-93-3View Details
P-Nitrobiphenyl 97% CAS 92-93-3View Details
92-93-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
