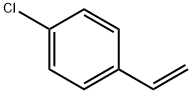
4-Methylstyrene
Synonym(s):4-Vinyltoluene
- CAS NO.:622-97-9
- Empirical Formula: C9H10
- Molecular Weight: 118.18
- MDL number: MFCD00008621
- EINECS: 210-762-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:07:02
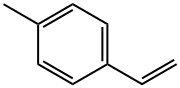
What is 4-Methylstyrene?
Chemical properties
CLEAR COLOURLESS TO LIGHT YELLOW LIQUID
The Uses of 4-Methylstyrene
4-Methylstyrene is used as a monomer for polyesters and in plastics production. It is also used as an intermediate in paint and coating additives. Further, it is used with other vinyltoluene isomers (3-vinyltoluene) as monomers for the preparation of poly(vinyltoluene). In addition to this, it is employed as a bi- ligand in the preparation of cationic, two-coordinate triphenylphosphine-gold(I)-pi complexes. Further, it is involved in Heck coupling reactions with chlorobenzene.
Definition
ChEBI: 4-Methylstyrene is a member of styrenes.
General Description
A clear colorless liquid with an aromatic odor. Flash point 129°F. Usually shipped with an inhibitor such as tert-butyl catechol added May polymerize if contaminated or subjected to heat. If polymerization takes place inside a closed container, the container may rupture violently. Vapors irritate the mucous membranes. Less dense than water and insoluble in water. Hence floats on water. Density is 7.6 lb / gal. Used in making plastics, especially as a monomer for polyesters.
Air & Water Reactions
Flammable. Insoluble in water.
Reactivity Profile
4-Methylstyrene may react vigorously with strong oxidizing agents. May react exothermically with reducing agents to release hydrogen gas. In the presence of various catalysts (such as acids) or initiators, may undergo exothermic addition polymerization reactions. May undergo autoxidation upon exposure to the air to form peroxides. These peroxides and polyperoxides are usually extremely unstable and liable to detonation. The peroxidation of butadiene has been involved in several serious industrial accidents.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control or dilution water may cause pollution.
Purification Methods
Purify it as the above styrenes and add a small amount of antioxidant if it is to be stored. It has UV in EtOH at max 285nm (log 3.07), and in EtOH + HCl 295nm (log 2.84) and 252nm (log 4.23). [Schwartzman & Carson J Am Chem Soc 78 322 1956, Joy & Orchin J Am Chem Soc 81 305 1959, Buck et al. J Chem Soc 23771949, Beilstein 5 IV 1369.]
Properties of 4-Methylstyrene
| Melting point: | -34 °C |
| Boiling point: | 170-175 °C(lit.) |
| Density | 0.897 g/mL at 25 °C(lit.) |
| vapor pressure | <1 mm Hg ( 20 °C) |
| refractive index | n |
| Flash point: | 114 °F |
| storage temp. | Store at <= 20°C. |
| solubility | 0.089g/l |
| form | Liquid |
| color | Clear colorless to light yellow |
| explosive limit | 1.1-5.2%(V) |
| Water Solubility | Miscible with benzene. slightly miscible with water. |
| BRN | 1209317 |
| CAS DataBase Reference | 622-97-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzene, 1-ethenyl-4-methyl-(622-97-9) |
| EPA Substance Registry System | p-Methyl styrene (622-97-9) |
Safety information for 4-Methylstyrene
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H226:Flammable liquids H304:Aspiration hazard H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P273:Avoid release to the environment. |
Computed Descriptors for 4-Methylstyrene
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 4-Methylstyrene, stabilized with 0.1% 3,5-di-tert-butylcatechol CAS 622-97-9View Details
4-Methylstyrene, stabilized with 0.1% 3,5-di-tert-butylcatechol CAS 622-97-9View Details
622-97-9 -
 4-Methylstyrene (stabilized with TBC) CAS 622-97-9View Details
4-Methylstyrene (stabilized with TBC) CAS 622-97-9View Details
622-97-9 -
 4-Methylstyrene CAS 622-97-9View Details
4-Methylstyrene CAS 622-97-9View Details
622-97-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1