4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture)
Synonym(s):(+)-Rose oxide;(4R)-2-(2-Methyl-1-propenyl)-4-methyltetrahydropyran;(4S)-2-(2-Methyl-1-propenyl)-4-methyltetrahydropyran
- CAS NO.:16409-43-1
- Empirical Formula: C10H18O
- Molecular Weight: 154.25
- MDL number: MFCD00036607
- EINECS: 240-457-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
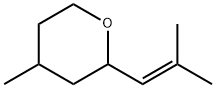
What is 4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture)?
Chemical properties
Rose Oxide occurs in small quantities, mainly as the levorotatory cis form, in essential oils (e.g., Bulgarian rose oil and geranium oil). Commercial synthetic products are either optically active or inactive mixtures of the cis- and trans-isomers. Their sensory properties depend on the starting material and the method of synthesis.They are colorless liquids with a strong odor reminiscent of geranium and rose oil.
Chemical properties
Tetrahydro-4-methyl-2-(2-methylpropen-1-yl)pyran has a powerful distinctive geranium top note.
Occurrence
Reported found in the oils of rose (Bulgarian) and geranium (Réunion); both the cis- and the trans-form have been reportedly isolated from geranium oil. Unidentified isomers reported found in black currant berries, passion fruit, some types of Thymus and white wine.
Definition
ChEBI: Rose oxide is a member of the class of oxanes that is tetrahydro-2H-pyran which is substituted at positions 2 and 4 by an isoprop-1-enyl group and a methyl group, respectively. Organic compound of the pyran class and the monoterpene class and a fragrance found in roses and rose oil. All four possible stereoisomers are known; the 2S,4R and 2S,4S diastereoisomers [also known as the (-)-cis- and (-)-trans-isomers, respectively] are the main constituents in several essential oils and are used as a food flavouring and in perfumes and cosmetics. It has a role as a plant metabolite. It is a monoterpenoid, a member of oxanes and an olefinic compound.
Preparation
Rose oxide is usually prepared from citronellol, which can be converted into a
mixture of two allyl hydroperoxides by photosensitized oxidationwith singlet oxygen.
Reduction of the hydroperoxideswith sodium sulfite yields the corresponding
diols . Treatmentwith dilute sulfuric acid results in allylic rearrangement and
spontaneous cyclization of one of the isomers; amixture of diastereoisomeric rose
oxides is thus formed. The unreacted diol isomer is separated by distillation. (?)-
Citronellol as the starting material yields an approximately 7 : 3 mixture of (?)-cisand
(?)-trans-rose oxide. Higher proportions of the cis-isomer may be obtained
by dehydration with a stronger acid.Other methods for the production of rose oxide starting from citronellol consist
of halogenation–dehalogenation reactions of citronellol.
An alternative technical process does not use citronellol but starts from prenal
[107-86-8] and 3-methyl-3-buten-1-ol [763-32-6]. Under acidic conditions,
the reaction affords a mixture of isomers of dehydro rose oxide (C10H16O6; [1786-
08-9], [92356-05-3], [60857-05-8]). Subsequent hydrogenation using a
ruthenium/alumina catalyst leads to rose oxide.The proportion of cis-isomer can
be increased by isomerization under acidic conditions.
Taste threshold values
Taste characteristics at 20 ppm: green, vegetative and herbal with a citrus nuance.
Flammability and Explosibility
Non flammable
Properties of 4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture)
| Boiling point: | 86 °C20 mm Hg(lit.) |
| Density | 0.873 g/mL at 20 °C(lit.) |
| vapor pressure | 53Pa at 20℃ |
| FEMA | 3236 | TETRAHYDRO-4-METHYL-2-(2-METHYLPROPEN-1-YL)PYRAN |
| refractive index | n |
| Flash point: | 157 °F |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| form | neat |
| color | A colourless mobile liquid. |
| Odor | at 1.00 % in dipropylene glycol. green red rose spicy fresh geranium |
| optical activity | [α]20/D 28±1°, neat |
| Water Solubility | 920mg/L at 20℃ |
| JECFA Number | 1237 |
| BRN | 1680588 |
| CAS DataBase Reference | 16409-43-1(CAS DataBase Reference) |
| EPA Substance Registry System | 2H-Pyran, tetrahydro-4-methyl-2-(2-methyl-1-propenyl)- (16409-43-1) |
Safety information for 4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture)
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. |
Computed Descriptors for 4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture)
4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture) manufacturer
Suru Chemicals And Pharmaceuticals Pvt Ltd
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 4-Hydrazinobenzoic acid 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 4-methoxy-3,5-dinitropyridine 2-(Cyanocyclohexyl)acetic acid 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 16409-43-1 ROSE OXIDE 98%View Details
16409-43-1 ROSE OXIDE 98%View Details
16409-43-1 -
 16409-43-1 98%View Details
16409-43-1 98%View Details
16409-43-1 -
 Rose oxide 98%View Details
Rose oxide 98%View Details
16409-43-1 -
 Rose oxide 16409-43-1 98%View Details
Rose oxide 16409-43-1 98%View Details
16409-43-1 -
 4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture) CAS 16409-43-1View Details
4-Methyl-2-(2-methyl-1-propenyl)tetrahydropyran (cis- and trans- mixture) CAS 16409-43-1View Details
16409-43-1 -
 (−)-Rose oxide CAS 16409-43-1View Details
(−)-Rose oxide CAS 16409-43-1View Details
16409-43-1 -
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0