4-Vinylphenol
- CAS NO.:2628-17-3
- Empirical Formula: C8H8O
- Molecular Weight: 120.15
- MDL number: MFCD00017593
- EINECS: 220-103-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-19 17:28:17
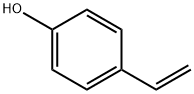
What is 4-Vinylphenol?
Chemical properties
4-Hydroxystyrene, also known as 4-vinylphenol or p-vinylphenol, is a colorless liquid with a vanilla extract odor. It can be dissolved in various organic solvents such as ethanol, acetone, benzene, and dimethyl sulfoxide, but it does not dissolve in water. This compound exhibits good stability, although it can be affected by light and ozone exposure, leading to potential oxidization reactions. It is an important chemical used as a monomer for producing poly(4-vinylphenol) (PVPh).
Occurrence
4-Vinylphenol is found in wine and beer. It is produced by the spoilage yeast Brettanomyces. When it reaches concentrations greater than the sensory threshold, it can give the wine aromas described as barnyard, medicinal, band-aids, and mousy. In wine, 4-vinylphenol can react with other molecules, such as anthocyanidins, to produce new chemical compounds. In white wines vinylphenols are dominant (4-vinylphenol 70-1 150 μg/l, 4-vinylguaiacol 10-490 μg/l) whereas, in red wines, it is the corresponding ethyl phenols.
Reported found in apple, cooked asparagus, dried bonito fish, cloudberries, cooked corn, roasted peanuts, rice bran, sandalwood, wild strawberries, vetiver oil, cooked apple, black currant, red wine, white wine, rose wine, coffee, green tea cognac, tomato, partially fermented tea, microbial fermented tea, black tea, heated soybean, coriander seeds, beer, cognac, peanut butter, soybean, beans, mushrooms, starfruit, tamarind, mango, rice, sweet corn, corn oil, malt, wort, rosemary and Bourbon vanilla.
The Uses of 4-Vinylphenol
4-Vinylphenol, 10 wt.% In Propylene Glycol can be used as a perfume oil mixture.
What are the applications of Application
4-Vinylphenol is a useful phenol compound
Definition
ChEBI: 4-hydroxystyrene is a member of the class of phenols that is styrene carrying a hydroxy substituent at position 4. It has a role as a human urinary metabolite and a human xenobiotic metabolite. It derives from a hydride of a styrene.
Production Methods
4-Hydroxystyrene (mp 73.5 C, sublimation range 70–80 ℃ at 0.004 kPa) can be obtained by gas-phase dehydrogenation of 4- ethylphenol at 550–600 ℃ and 0.1 MPa on an iron oxide catalyst and in the presence of steam and an aromatic hydrocarbon as diluents. The alkenylphenol, which polymerizes readily, can be separated from the less acidic unconverted 4-ethylphenol by extracting the condensed reactor effluent with alkali at 30 ℃.
What are the applications of Application
4-Vinylphenols, a class of functionalized styrenes, constitute one of the most extensively explored compounds due to their wide-ranging applications in food and alcoholic beverages, flavoring substances, and as intermediates in the preparation of various bioactive molecules, polymers, and copolymers that are useful in coatings, electronic applications, ion exchange resins, and photoresists, etc. In addition, several biological activities, such as antioxidant, antibacterial, antifungal, hypolipidemic, and antimutagenic, have been attributed to these compounds[1].
Aroma threshold values
Detection: 10 to 85 ppb
Taste threshold values
Taste characteristics at 60 ppm: phenolic, medicinal with sweet musty and meaty nuances.
Flammability and Explosibility
Not classified
References
[1] Arun K. Sinha, Bhupendra P. Joshi, Anuj Sharma. “One-Pot Two-Step Synthesis of 4-Vinylphenols from 4-Hydroxy Substituted Benzaldehydes under Microwave Irradiation: A New Perspective on the Classical Knoevenagel—Doebner Reaction.” ChemInform 38 19 (2007).
Properties of 4-Vinylphenol
| Melting point: | 73 °C |
| Boiling point: | 228.85°C (estimate) |
| Density | 1.04 |
| vapor pressure | 9.38hPa at 25℃ |
| refractive index | 1.4440 |
| FEMA | 3739 | P-VINYLPHENOL |
| Flash point: | >230 °F |
| storage temp. | under inert gas (nitrogen or Argon) at 2-8°C |
| solubility | Chloroform (Sparingly), DMSO (Slightly) |
| form | Liquid |
| pka | 9.95±0.26(Predicted) |
| color | Clear colorless to orange |
| Odor | at 0.01 % in propylene glycol. chemical phenolic medicinal sweet |
| Water Solubility | Slightly miscible with water. |
| Sensitive | Hygroscopic |
| JECFA Number | 711 |
| BRN | 506844 |
| Stability: | Light Sensitive |
| CAS DataBase Reference | 2628-17-3(CAS DataBase Reference) |
| NIST Chemistry Reference | 4-hydroxystyrene(2628-17-3) |
| EPA Substance Registry System | Phenol, 4-ethenyl- (2628-17-3) |
Safety information for 4-Vinylphenol
| Signal word | Warning |
| Pictogram(s) |
 Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 4-Vinylphenol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 4-Vinylphenol CAS 2628-17-3View Details
4-Vinylphenol CAS 2628-17-3View Details
2628-17-3 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
