4-HYDROXY PROPRANOLOL HCL
- CAS NO.:14133-90-5
- Empirical Formula: C16H21NO3.ClH
- Molecular Weight: 311.8
- MDL number: MFCD06656370
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-18 11:31:05
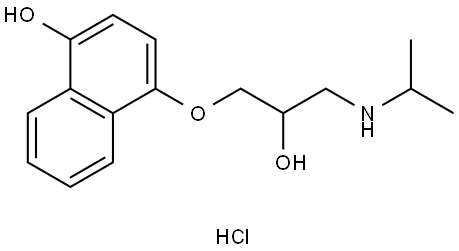
What is 4-HYDROXY PROPRANOLOL HCL?
Chemical properties
Off-White Solid
The Uses of 4-HYDROXY PROPRANOLOL HCL
The main metabolite of (R)-Propranolol
The Uses of 4-HYDROXY PROPRANOLOL HCL
The main metabolite of (S)-Propranolol
The Uses of 4-HYDROXY PROPRANOLOL HCL
A metabolite of Propranolol.
What are the applications of Application
(±)-4-Hydroxy Propranolol Hydrochloride is a metabolite of Propranolol
Biological Activity
(±)-4-hydroxy propranolol, an active metabolite of propranolol, blocks β1- and β2-adrenergic receptors (β1-ars, β2-ars). also, (±)-4-hydroxy propranolol has antioxidant properties at micromolar concentrations. β1- and β2-ars, expressed in cardiac myocytes, mediate an increase in contractility by gs-dependent coupling to adenylyl cyclase.
in vitro
compared to the control, (±)-4-hydroxy propranolol potently blocked the lipid peroxidation in a concentration-dependent fashion in endothelial cells. when pretreated with (±)-4-hydroxy propranolol at 0.067 to 6.7 μm, the degrees of protection were increased against the glutathione loss in the endothelial cells. additionally, (±)-4-hydroxy propranolol effectively preserved the loss of cell survival because of the radical stress [1].
in vivo
rats were injected intravenously with (±)-4-hydroxy propranolol into the femoral vein at 0.1 ml/100 g. (±)-4-hydroxy propranolol induced an increase in heart rate in a dose-dependent manner in rats depleted of catecholamines, which suggested that (±)-4-hydroxy propranolol had intrinsic sympathomimetic activity. the response of (±)-4-hydroxy propranolol was inhibited when rats were pretreated with 0.5 mg/kg propranolol [2].
References
[1]. mak, i. potent antioxidant properties of 4-hydroxyl-propranolol. journal of pharmacology and experimental therapeutics. 2003; 308(1): 85-90.
[2]. fitzgerald, j., & o'donnell, s. pharmacology of 4-hydroxypropranolol, a metabolite of propranolol. british journal of pharmacology. 1971; 43(1): 222-235.
Properties of 4-HYDROXY PROPRANOLOL HCL
| Melting point: | 156-158°C |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| color | Pale Red to Black |
| Stability: | Hygroscopic |
Safety information for 4-HYDROXY PROPRANOLOL HCL
Computed Descriptors for 4-HYDROXY PROPRANOLOL HCL
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 (±)-4-Hydroxypropranolol hydrochloride CAS 14133-90-5View Details
(±)-4-Hydroxypropranolol hydrochloride CAS 14133-90-5View Details
14133-90-5 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1