Islatravir
- CAS NO.:865363-93-5
- Empirical Formula: C12H12FN5O3
- Molecular Weight: 293.25
- MDL number: MFCD28502143
- Update Date: 2024-11-19 23:02:33
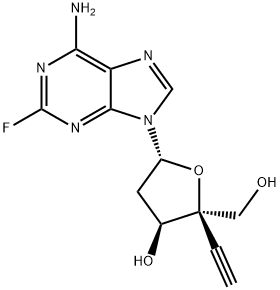
What is Islatravir?
Description
Islatravir belongs to a group of HIV drugs called nucleoside reverse transcriptase translocation inhibitors (NRTTIs). NRTTIs use several different methods to block an HIV enzyme called reverse transcriptase. By blocking reverse transcriptase, NRTTIs prevent HIV from multiplying and can reduce the amount of HIV in the body. This compound may be effective against certain HIV strains that are resistant to other HIV drugs.
The Uses of Islatravir
4'-ethynyl-2-fluoro-2'-deoxyadenosine is a heterocyclic organic compound and can be used as pharmaceutical intermediates.
The Uses of Islatravir
MK-?8591 is a carbocyclic nucleoside reverse transcriptase translocation inhibitor and is used as an antiviral with potential use towards the treatment of HIV.
Mechanism of action
Islatravir belongs to a group of HIV drugs called nucleoside reverse transcriptase translocation inhibitors (NRTTIs). NRTTIs use several different methods to block an HIV enzyme called reverse transcriptase. By blocking reverse transcriptase, NRTTIs prevent HIV from multiplying and can reduce the amount of HIV in the body. This compound may be effective against certain HIV strains that are resistant to other HIV drugs[1].
Pharmacokinetics
Islatravir is converted intracellularly by endogenous kinases to its active triphosphate (TP) form, islatravir-TP, which has a long intracellular half-life, allowing the potential for various dosing options and regimens. In participants without HIV, single oral doses of islatravir were rapidly absorbed, with peak plasma concentrations attained at ~ 0.5 h, with islatravir demonstrating a half-life of ~ 49–61 h. Peak intracellular islatravir-TP concentrations were attained 6–24 h after islatravir administration, with a half-life of 118–171 h; similar pharmacokinetics have been observed in people living with HIV-1. When administered once daily (QD), oral doses of islatravir resulted in steady-state plasma concentrations at approximately Day 14. At the same time, intracellular islatravir-TP reached a steady state by Day 28 and remained above the concentration threshold associated with antiviral efficacy (0.05 pmol/106 cells) for ~ 30 days following cessation of dosing. In addition, doses as low as 0.25 mg QD produced intracellular islatravir-TP concentrations exceeding the therapeutic concentration threshold on Day 1[1].
References
[1] Randolph P Matthews. “A Phase 1 Study to Evaluate the Drug Interaction Between Islatravir (MK-8591) and Doravirine in Adults Without HIV.” Clinical Drug Investigation (2021): 629–638.
Properties of Islatravir
| Melting point: | 220.0-221.4 °C (decomp) |
| Boiling point: | 669.2±65.0 °C(Predicted) |
| Density | 1.72±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:100.0(Max Conc. mg/mL);341.0(Max Conc. mM) Water:3.57(Max Conc. mg/mL);12.17(Max Conc. mM) |
| form | A solid |
| pka | 13.11±0.60(Predicted) |
| color | White to off-white |
| InChI | InChI=1S/C12H12FN5O3/c1-2-12(4-19)6(20)3-7(21-12)18-5-15-8-9(14)16-11(13)17-10(8)18/h1,5-7,19-20H,3-4H2,(H2,14,16,17)/t6-,7+,12+/m0/s1 |
Safety information for Islatravir
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Islatravir
| InChIKey | IKKXOSBHLYMWAE-QRPMWFLTSA-N |
| SMILES | OC[C@@]1(C#C)O[C@@H](N2C3C(=C(N=C(F)N=3)N)N=C2)C[C@@H]1O |
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Mefenamic Acid IP/BP/EP/USP Diclofenac Sodium IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4