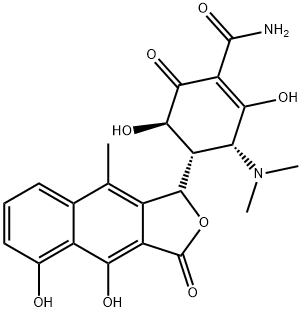
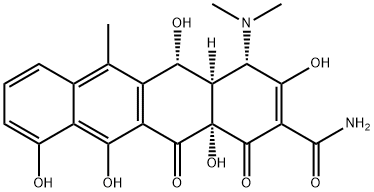
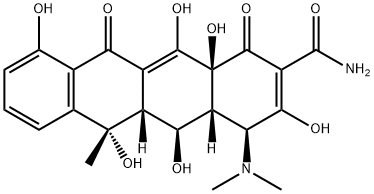
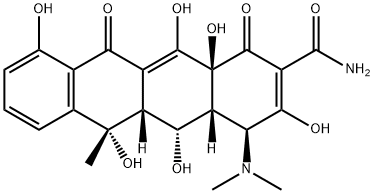
4-Epioxytetracycline
- CAS NO.:14206-58-7
- Empirical Formula: C22H24N2O9
- Molecular Weight: 460.43
- MDL number: MFCD00021181
- EINECS: 204-888-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
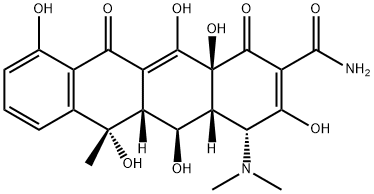
What is 4-Epioxytetracycline?
Description
Epioxytetracycline is a degradation product of the antibiotic oxytetracycline . It has been found in swine manure compost and wastewater and is considered a pollutant.
Chemical properties
greenish-beige to brownish powder
The Uses of 4-Epioxytetracycline
A metabolite of Oxytetracycline
The Uses of 4-Epioxytetracycline
Epioxytetracycline is a degradation product of oxytetracycline formed by the epimerisation of the dimethylamino group at C4 in solution at neutral to acidic pH. The epimerisation is associated with a loss of antibiotic activity. Epioxytetracycline is an important standard for monitoring oxytetracycline stability.
What are the applications of Application
4-Epioxytetracycline is a metabolite of Oxytetracycline (OTC)
Mode of action
Oxytetracycline causes inhibition of protein synthesis. It binds to the 30S ribosomal subunit and prevents the amino-acyl tRNA from binding to the A site of the ribosome.
In an acellular model system of protein synthesis using ribosomes from tetracycline-sensitive and resistant strains of E. coli in the synthesis of polyphenylalanine, Oxytetracycline showed inhibitory activity along with minocycline. 4-Epioxytetracycline and beta-apo-oxytetracycline had competing properties with respect to oxytetracycline at the stage of penetration through the cell membrane but did not, however, suppress the synthesis of polyphenylalanine.
References
1] I V BELIAVSKAIA. [Study of the mechanism of action of minocycline and of certain other tetracycline group compounds].[J]. Antibiotiki, 1976, 21 3: 242-245.
[2] BENT HALLING-S?RENSEN J. T G Sengel?v. Toxicity of Tetracyclines and Tetracycline Degradation Products to Environmentally Relevant Bacteria, Including Selected Tetracycline-Resistant Bacteria[J]. Archives of Environmental Contamination and Toxicology, 2002, 42 1: 263-271. DOI:10.1007/s00244-001-0017-2.
[3] ANNE KRUSE LYKKEBERG . Quantitative analysis of oxytetracycline and its impurities by LC-MS-MS[J]. Journal of pharmaceutical and biomedical analysis, 2004, 34 2: Pages 325-332. DOI:10.1016/S0731-7085(03)00500-4.
[4] RICHENG XUAN. Hydrolysis and photolysis of oxytetracycline in aqueous solution.[J]. Journal of Environmental Science and Health Part B-pesticides Food Contaminants and Agricultural Wastes, 2010, 45 1: 73-81. DOI:10.1080/03601230903404556.
[5] HONGXING HAN. Impact of 4-epi-oxytetracycline on the gut microbiota and blood metabolomics of Wistar rats[J]. Scientific Reports, 2016, 6 1. DOI:10.1038/srep23141.
Properties of 4-Epioxytetracycline
| Melting point: | 168°C |
| Boiling point: | 839.6±65.0 °C(Predicted) |
| Density | 1.71±0.1 g/cm3(Predicted) |
| storage temp. | Hygroscopic, -20°C Freezer, Under Inert Atmosphere |
| solubility | DMSO: slightly soluble; Methanol: slightly, heated |
| pka | 4.50±1.00(Predicted) |
| form | Powder |
| color | Greenish-beige to brownish |
| Stability: | Hygroscopic, Light Sensitive, Unstable in Solution |
| EPA Substance Registry System | 2-Naphthacenecarboxamide, 4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,6,10,12,12a-hexahydroxy-6-methyl-1,11-dioxo-, (4R,4aR,5S,5aR,6S,12aS)- (14206-58-7) |
Safety information for 4-Epioxytetracycline
Computed Descriptors for 4-Epioxytetracycline
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Guanine , 99%View Details
Guanine , 99%View Details
73-40-5 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Potassium Hydroxide 90%View Details
Potassium Hydroxide 90%View Details
1310-58-3 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
